Ammonia (NH3) plays a pivotal role in contemporary agriculture and energy industries. As a primary ingredient in fertilizers, ammonia contributes to global food security. However, its advantages extend beyond agriculture, as it is also being recognized as a clean and efficient energy carrier. Due to its high energy density, ammonia can potentially serve as a zero-carbon fuel, offering a valuable alternative to fossil fuels with its clean combustion products. Nonetheless, traditional methods of ammonia production, primarily the Haber-Bosch process, are energy-intensive and significantly contribute to global carbon emissions, accounting for around 1.8% of the total emissions. Consequently, the quest for more sustainable and efficient ammonia synthesis methods is garnering increasing attention from researchers worldwide.
Recent research published in ACS Nano has made headway in the development of catalysts for the electrochemical nitrate reduction reaction (eNO3RR) to ammonia. This innovative approach not only addresses the sustainability of ammonia production but also offers a pathway to convert nitrate wastes into a usable form, presenting a dual benefit of reducing environmental pollutants while generating a valuable product. The research focuses on spinel cobalt oxides (Co3O4), which have emerged as attractive candidates for eNO3RR due to their affordability, enhanced catalytic performance, and high selectivity compared to traditional catalysts.
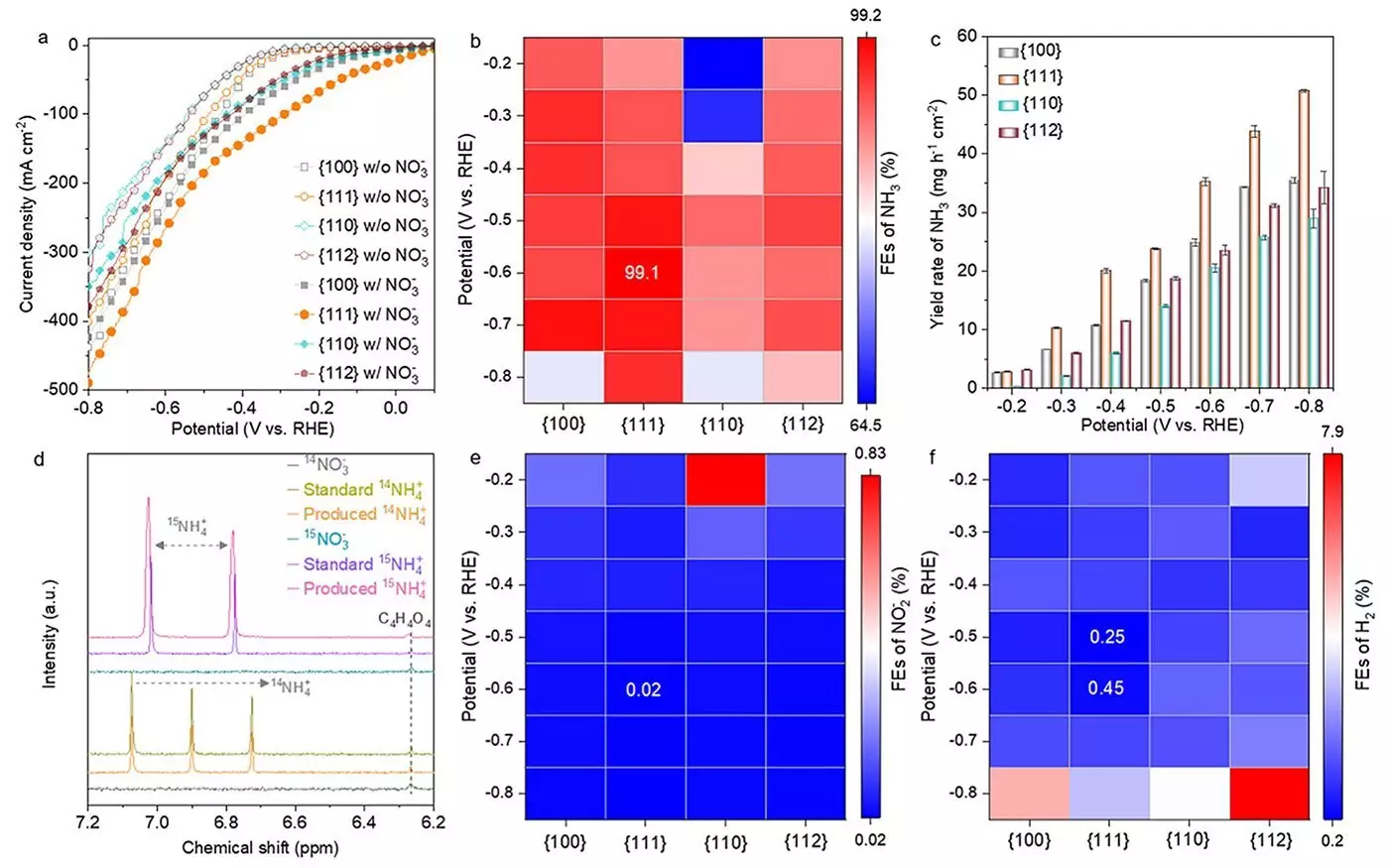
The researchers critically examined various Co3O4 nanostructures having different crystallographic facets including {100}, {111}, {110}, and {112}. These facets influence the reaction dynamics and efficiency for ammonia production. Notably, the {111} facet demonstrated remarkable results, achieving a Faradaic efficiency of 99.1% and producing an impressive yield rate of 35.2 mg h-1 cm-2. This finding elucidates the significance of facet-oriented materials, catalyzing advancements in the field of electrocatalysis.
A notable aspect of this research is the investigation of the transformation process of the Co3O4 catalyst during nitrate reduction. The team observed a succession of structural changes that included the evolution from Co3O4 to an intermediate structure with oxygen vacancies, ultimately stabilizing as Co(OH)2. This transformational journey was particularly pronounced on the {111} facet, underscoring its superiority in facilitating the conversion of nitrate into ammonia. Dr. Heng Liu, a contributing researcher, emphasized this catalytic enhancement due to the rapid formation of oxygen vacancies and the conversion to Co(OH)2 on this facet.
These insights into the catalyst’s structural transformations reveal a significant area of interest for future studies. Understanding the underlying mechanisms that govern catalyst activity is crucial in advancing the field, as it opens pathways for creating more efficient catalysts designed for specific purposes.
The implications of successfully implementing eNO3RR are outstanding, particularly in the context of global environmental goals. As nations strive to achieve carbon neutrality by mid-century, the development of cleaner processes in critical industries becomes essential. This research not only demonstrates the potential for the electrochemical conversion of nitrate waste into ammonia but also highlights a sustainable means of dealing with excess nitrates commonly found in agricultural runoff.
Moreover, the evolution of ammonia as an energy carrier could help to decarbonize various sectors, linking agricultural practices with advancements in energy storage and conversion technologies. The ongoing research aims to control the final phases of the catalyst’s transformation, guaranteeing improvements in activity, selectivity, and overall stability.
The work accomplished in optimizing Co3O4 catalysts represents a significant step forward in sustainable ammonia production. By enhancing the understanding of the structural behaviors of catalysts, researchers are now equipped to design strategies that optimize performance and mitigate environmental impacts. As the world modernizes, transitioning toward more sustainable industrial processes must remain a priority. The findings from this study indeed have the potential to revolutionize ammonia production, setting a solid foundation for a greener future where agricultural needs and energy demands can coexist harmoniously with environmental sustainability.