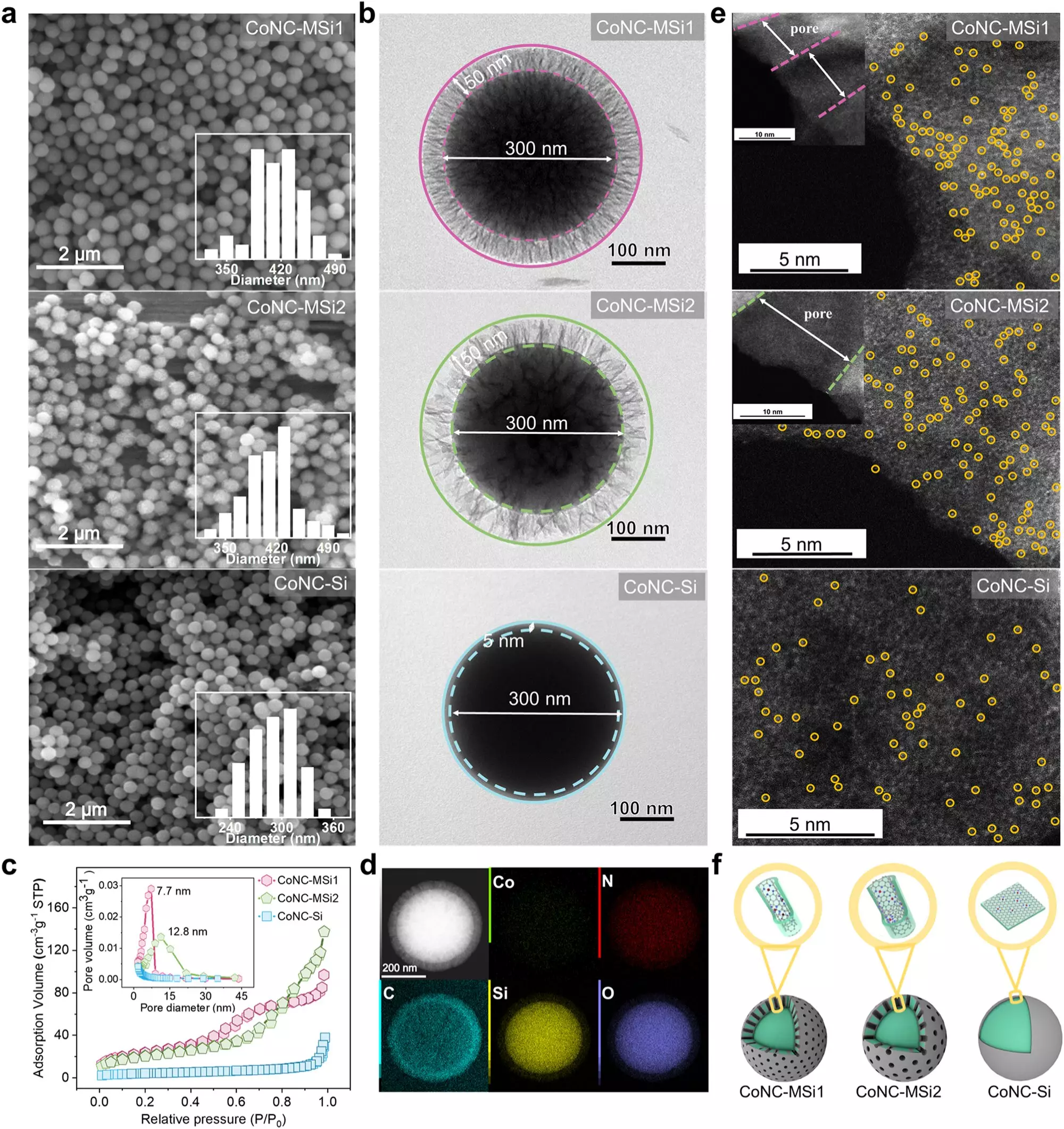
In the ongoing battle against water pollution, a groundbreaking advancement has emerged from researchers affiliated with the University of Science and Technology of China and the Suzhou Institute for Advanced Study. Their findings, recently published in *Nature Communications*, shed light on an innovative water purification technique utilizing single-atom catalysts (SACs) within a Fenton-like catalytic framework. This novel approach has the potential to significantly enhance the breakdown of harmful pollutants in aquatic environments.
Single-atom catalysts are essentially minuscule agents designed to facilitate chemical reactions with remarkable efficiency. However, the application of SACs in purifying water had faced certain limitations, primarily due to the restricted movement of reactants towards the catalyst’s surface and the excessive amounts of oxidants required to initiate the purification process. Previous studies had recognized some improvements in efficiency when utilizing nanoconfined SACs. Still, the precise mechanisms behind these enhancements were not entirely clear until now.
The research team’s pivotal discovery lies in the confining of SACs within exceedingly small pores in silica particles, expanding the potential for effective pollutant degradation. This method not only accelerates the reaction but also makes oxidant usage remarkably efficient. The researchers observed a significant transformation in the reaction pathway; instead of depending on traditional singlet oxygen, which is a reactive yet less efficient form of oxygen for this purpose, the process transitioned to a direct electron transfer mechanism. This shift markedly improves the degradation of pollutants, pushing the boundaries of conventional water purification technologies.
The study yielded startling results, illustrating a staggering 34.7-fold enhancement in the rate of pollutant degradation compared to traditional approaches. In addition, the efficiency of oxidant utilization rose strikingly from 61.8% to 96.6%, demonstrating not only the efficacy of the new method but also its potential applicability in real-world scenarios. Various electron-rich phenolic compounds—common contaminants in water sources—were effectively degraded, underscoring the robustness of this innovative solution across diverse environmental conditions.
The implications of this research extend far beyond laboratory settings. The findings provide a coherent understanding of how nanoconfined catalysts operate, paving the way for the potential advancement of eco-friendly water purification technologies. Both industrial and residential applications beckon, as the need for low-carbon, effective treatment methods continues to escalate in our fight against water pollution. Consequently, this research not only heralds a new chapter in advanced oxidation processes but also inspires further exploration of innovations in environmental science, addressing one of the most pressing issues faced globally today.
The fusion of single-atom catalysts with cutting-edge nanotechnology presents an exciting frontier in the realm of water purification, with promising applications on the horizon for cleaner, safer water systems worldwide.