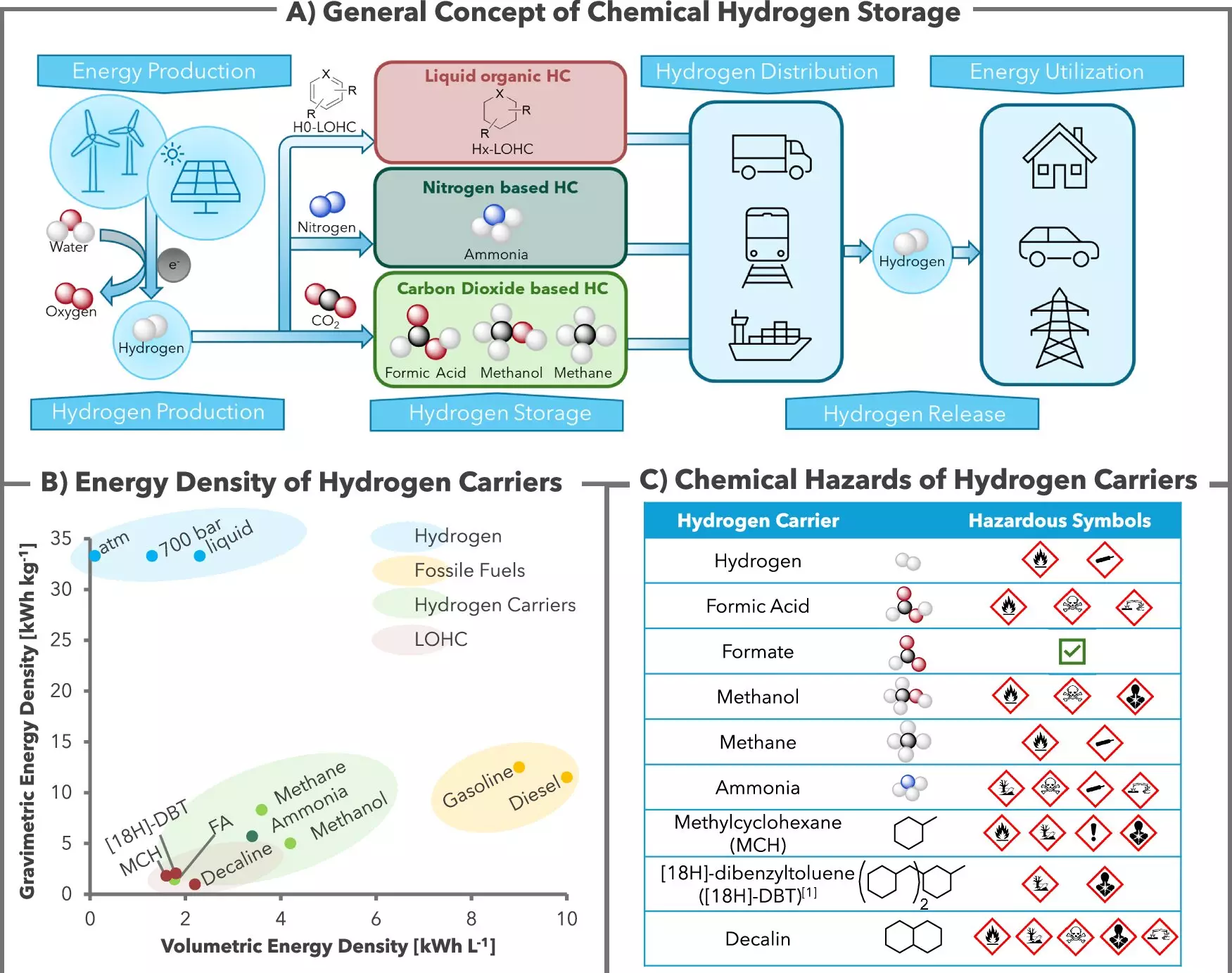
As the world transitions toward greener energy solutions, scientists are grappling with the complexities of safely and effectively storing hydrogen, a promising fuel that could revolutionize energy consumption. Researchers at the Leibniz Institute for Catalysis (LIKAT) in Rostock and the company H2APEX are taking significant steps in this area. Their recent study, published in the journal Nature Communications, unveils a novel catalyst system that employs common ingredients like potassium bicarbonate to chemically bind and store hydrogen. This breakthrough not only addresses concerns about hydrogen’s volatile nature but also improves its availability for various applications in green energy.
At the heart of this new approach is a reversible reaction facilitated by a ruthenium-based catalyst. When hydrogen (H2) encounters potassium bicarbonate, it reacts to form formate—the salt of formic acid—which safely sequesters the hydrogen. The ingenious aspect of this system is its reversibility; the stored hydrogen can be released and re-bound without consuming the catalyst. According to the researchers involved, this stability occurs at approximately 60 degrees Celsius, where the reaction occurs in a solution containing all necessary components: hydrogen, bicarbonate, and the catalyst. Notably, this method minimizes waste and enhances hydrogen purity—attributes critical for advancing hydrogen fuel cell technologies.
Hydrogen, while promising, poses storage and handling challenges due to its flammability and low density. Traditional methods of hydrogen storage, such as pressurized gas tanks or liquid hydrogen, can be both expensive and hazardous. The potassium bicarbonate system offers an alternative that is both cost-effective and safer. The researchers have highlighted that the formate solution can be managed similarly to common liquids like milk or beer in terms of infrastructure, showcasing the potential for practical applications in various sectors, including rural areas where renewable energy sources can be harnessed to produce green hydrogen.
One of the most compelling aspects of the LIKAT and H2APEX research is the CO2-neutral nature of the process. Typically, methods of hydrogen recovery generate carbon dioxide as a byproduct, complicating efforts to reduce greenhouse gas emissions. The team’s approach ensures that CO2 remains captured as part of the bicarbonate rather than being released into the atmosphere, allowing for environmentally friendly hydrogen production. This advancement contributes to the credibility of hydrogen as an alternative energy source and aligns with global sustainability goals.
A critical choice in this innovation is the selection of potassium bicarbonate as the storage medium. This salt boasts multiple advantages, including higher solubility and stability compared to conventional storage salts. In developing this method, the researchers evaluated various candidates but concluded that potassium offered a favorable balance of properties necessary for efficient hydrogen binding. Unlike sodium bicarbonate commonly found in kitchens, potassium bicarbonate serves a dual purpose in both energy generation and as a food-safe ingredient, showcasing its versatility.
The implications of this research extend beyond academia, with H2APEX poised to develop a larger-scale demonstrator aimed at commercializing the technology. By leveraging the findings from this study, including a remarkable 40 consecutive cycles of hydrogen storage and release with minimal catalyst use, the company is gearing up to transform the hydrogen economy. If successful, commercial systems could be ready as soon as 2025, ushering in a new era of hydrogen utilization as not just an energy source, but a cornerstone for sustainable industrial practices.
The collaborative efforts of LIKAT and H2APEX represent a meaningful stride towards sustainable hydrogen storage. As society pivots away from fossil fuels, this novel approach encapsulates both innovation and the promise of cleaner energy solutions. The implications of storing hydrogen in such a manageable form cannot be overstated, with the potential to fundamentally alter our energy landscape. As researchers refine these technologies, the chemical symbol for hydrogen (H) may come to symbolize not only the element itself but also the hope it represents for a cleaner, more sustainable energy future.