Fuel cells have emerged as a promising technology in the quest for sustainable energy solutions. They provide an efficient means of generating electricity through electrochemical reactions, eliminating the need for combustion and thus minimizing environmental pollution. This technology spans various applications, including electric vehicles, portable chargers, and industrial machinery. However, a significant barrier to the widespread adoption of fuel cells is the reliance on expensive materials and precious metal catalysts. In recent years, Anion-exchange-membrane fuel cells (AEMFCs) have offered a potential solution to this issue, utilizing more abundant and cost-effective materials.
Challenges Faced by Traditional Fuel Cells
The conventional fuel cell designs often grapple with the high costs associated with their catalysts. Many utilize precious metals such as platinum, leading to increased production costs and limited accessibility. This financial obstacle hinders large-scale implementation and restricts the technology’s growth potential. In response to these issues, researchers are increasingly focusing on AEMFCs, which promise lower costs by utilizing non-precious metal catalysts. However, the journey toward their practical application has been fraught with challenges, particularly concerning the durability and stability of these alternative materials.
Recent studies have shown that non-precious metals can suffer from self-oxidation, which leads to irreparable damage to the electrochemical cells. This degradation is a significant hurdle that needs to be addressed to make AEMFCs more commercially viable. The approach to overcoming these challenges is critical, as it could determine the future of fuel cell technology in a world striving for cleaner energy sources.
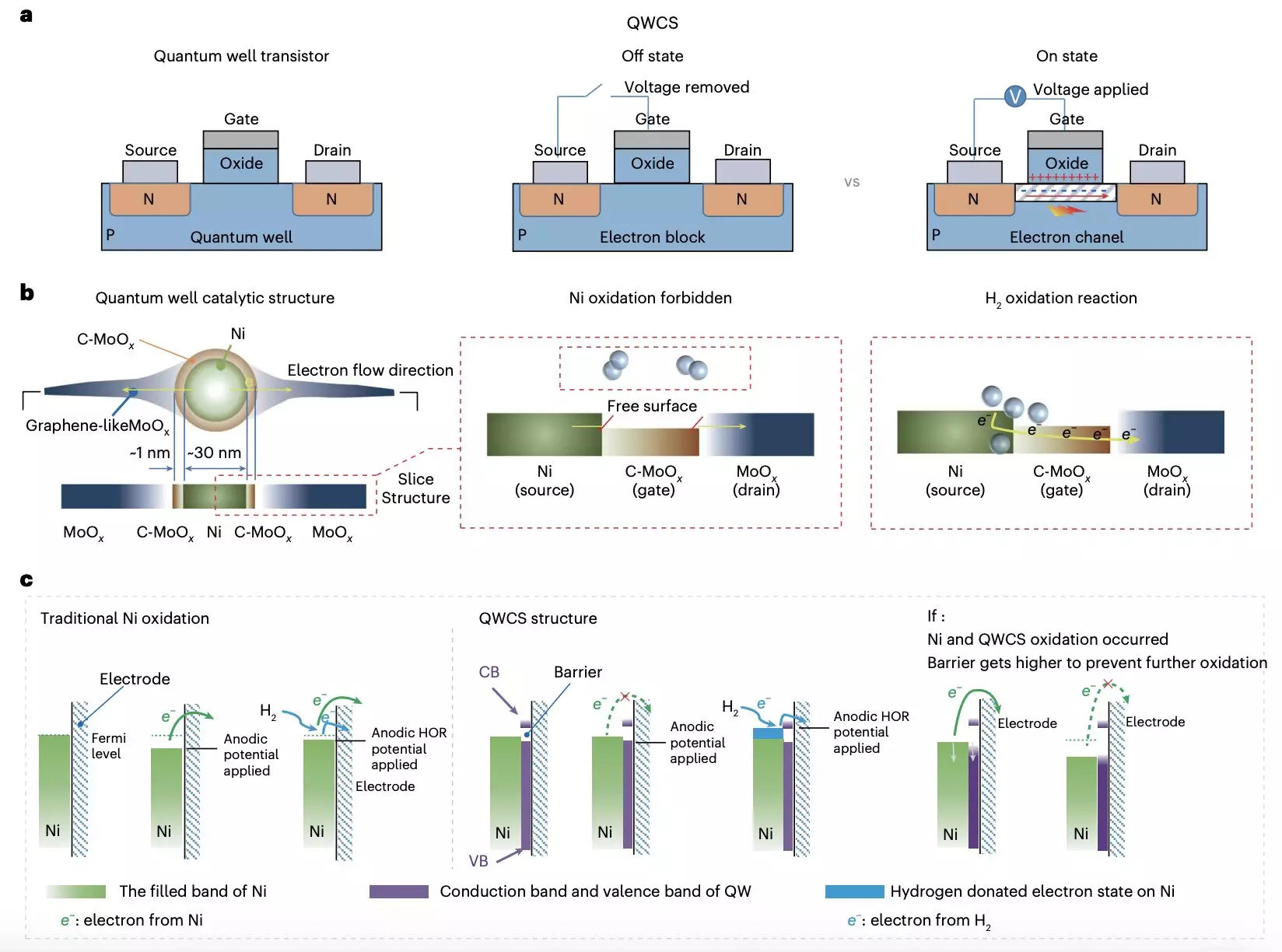
A groundbreaking study conducted by researchers at Chongqing University and Loughborough University has introduced an innovative method to enhance the performance of AEMFCs. By creating a quantum well-like catalytic structure (QWCS), the team has developed a unique strategy to prevent the oxidation of nickel-based electrocatalysts. This QWCS consists of quantum-confined metallic nickel nanoparticles that are atomically confined within a specific heterojunction that utilizes carbon-doped molybdenum oxide. The design aims to maintain the metallic state of the nickel catalyst while selectively transferring electrons produced during the hydrogen oxidation reaction.
This approach not only preserves the catalyst’s integrity but also enhances its performance under demanding conditions. The researchers aptly named the catalyst Ni@C-MoOx; it ensures that electrons produced via hydrogen oxidation efficiently cross the energy barrier without compromising the structural stability of the nickel nanoparticles. This innovative framework allows the catalyst to achieve a remarkable stability of 1.2 V against the reversible hydrogen electrode.
Promising Results and Future Implications
The implications of this research are profound. The Ni@C-MoOx catalyst maintained its electrochemical performance even after extensive testing, sustaining exceptional stability for over 100 hours under severe operational demands. This resilience positions it as a game-changer for the future of AEMFCs, offering an alternative that circumvents the degradation pitfalls associated with traditional non-precious metal catalysts.
The resulting AEMFC, which utilized this innovative catalyst, showcased a stellar power density of 486 mW/mgNi. Notably, the fuel cell exhibited consistent performance even after multiple shutdown and restart cycles, a testament to the robustness of the QWCS technology. In stark contrast, conventional AEMFCs without such protective barriers failed after just a single cycle.
As the demand for sustainable energy solutions continues to rise, breakthroughs like those achieved by the researchers at Chongqing University and Loughborough University could pave the way for safer, more efficient fuel cells. The novel QWCS design not only holds potential for enhancing the performance and reliability of AEMFCs but also opens avenues for developing other advanced catalysts utilizing quantum confinement principles.
The transition to a world dependent on cleaner energy sources is hindered by various challenges, but innovative approaches like QWCS and the research surrounding AEMFCs serve as vital steps towards overcoming these barriers. If adopted widely, technologies that leverage Earth-abundant materials might ultimately lead to affordable and efficient energy solutions that could change the landscape of energy consumption for generations to come.