Noble gases, long characterized by their chemical inertness, have historically been regarded as unreactive spectators in the pantheon of elemental chemistry. This preconceived notion faced a noteworthy challenge over 60 years ago when chemist Neil Bartlett successfully bonded xenon into a compound, producing xenon hexafluoroplatinate (XePtF6), which marked a groundbreaking moment in the field. Since then, the world of noble gas chemistry has expanded significantly, inspiring researchers to synthesize hundreds of noble gas compounds. However, the study of these compounds remains fraught with challenges, primarily due to their unstable nature when exposed to the atmosphere.
The Challenge of Crystallization
One of the most significant hurdles in the analysis of noble gas compounds lies in the crystallization process. The sensitivity of these compounds to moisture renders the growth of large, high-quality crystals extremely difficult. While single-crystal X-ray diffraction has allowed for some advances in understanding noble gas crystal structure, the general inability to produce sizable crystals has confined researchers’ ability to fully characterize many noble gas compounds. The result? A wealth of unexplored molecular insights tethered by the practical limitations of current analytical techniques.
Recent advancements have introduced new methodologies to address these inherent limitations. In particular, the development of 3D electron diffraction has opened new doors for the analysis of small, nanoscale crystals. This approach allows for the examination of tiny crystallites that retain stability in air, bridging a significant gap in the knowledge surrounding noble gas compounds. Researchers Lukáš Palatinus and Matic Lozinšek, alongside their team, took the initiative to apply this innovative technique specifically to xenon-containing compounds, seeking to unlock new insights from these elusive structures.
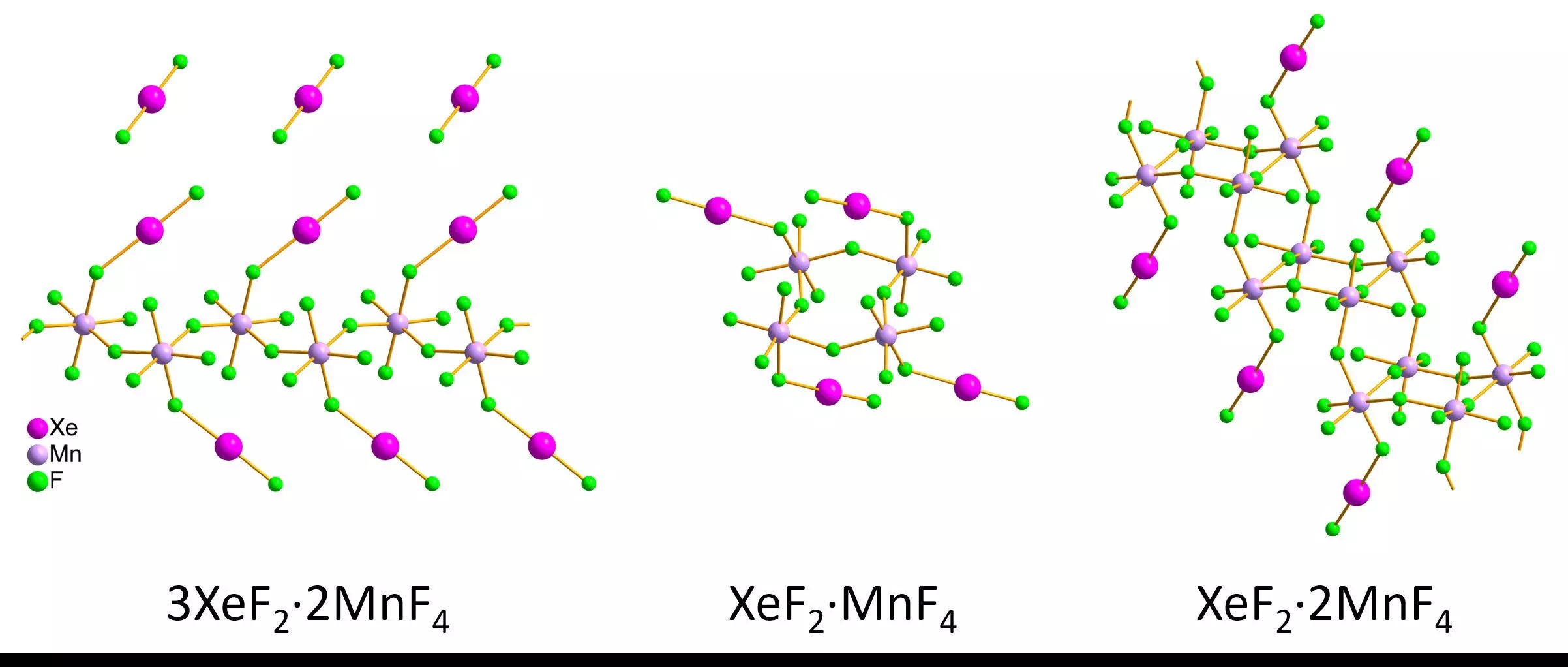
The research team synthesized three different xenon difluoride-manganese tetrafluoride compounds, yielding striking individual red crystals and pink powders. Remarkably, through a careful process of cooling and protective layering, the researchers transferred their samples for observation in a transmission electron microscope. Their meticulous technique ensured that the delicate structure of the xenon compounds remained intact, leading to detailed measurements of bond lengths and angles.
The comparative analysis between the smaller particles studied using 3D electron diffraction and previously characterized larger crystals with single-crystal X-ray diffraction demonstrated encouraging correlations. The findings revealed distinct structural motifs: infinite zigzag chains for 3XeF2·2MnF4, ring formations for XeF2·MnF4, and staircase-like configurations for XeF2·2MnF4. Such findings not only highlight the potential for 3D electron diffraction in studying air-sensitive substances, but they also enhance our understanding of noble gas involvement in complex molecular architectures.
Implications for Future Research
The successful application of 3D electron diffraction on xenon compounds signals a new era for noble gas research. This technique promises to demystify long-standing compounds, such as XePtF6, and to broaden the understanding of other air-sensitive substances, potentially leading to further breakthroughs in materials science and chemistry. By pushing the boundaries of classical techniques, this research exemplifies the endless pursuit of knowledge and the capability of modern science to unravel the complexities and hidden structures of even the most elusive chemical entities.