In a groundbreaking development, a team from the University of Illinois Urbana-Champaign has pioneered a novel polymer that could drastically alter the landscape of chemical separation. This innovative technology selectively attracts specific substances when electrically activated, marking a significant step toward sustainable and efficient methods for isolating chemicals. This article delves into the intricacies of this research and its pivotal role in shaping the future of chemical engineering.
Chemical separation has long been a cornerstone of industries that range from pharmaceuticals to environmental science. Traditional methods often rely on thermal processes or membrane filtration, both of which can generate substantial waste and require significant energy input. The introduction of a dynamic polymer that operates via electrochemical principles represents a paradigm shift. By applying electrical stimulation, the polymer is able to selectively bind certain ions and molecules—such as halides and oxyanions—while ignoring others.
Professor Xiao Su, the lead researcher on the project, drew an apt analogy comparing this new technology to a sponge that absorbs only desired chemicals from a mixture. The novelty lies not merely in its function but also in its selective capabilities, which were previously elusive in electrochemical separation techniques.
The Mechanism Behind the Electric Sponge
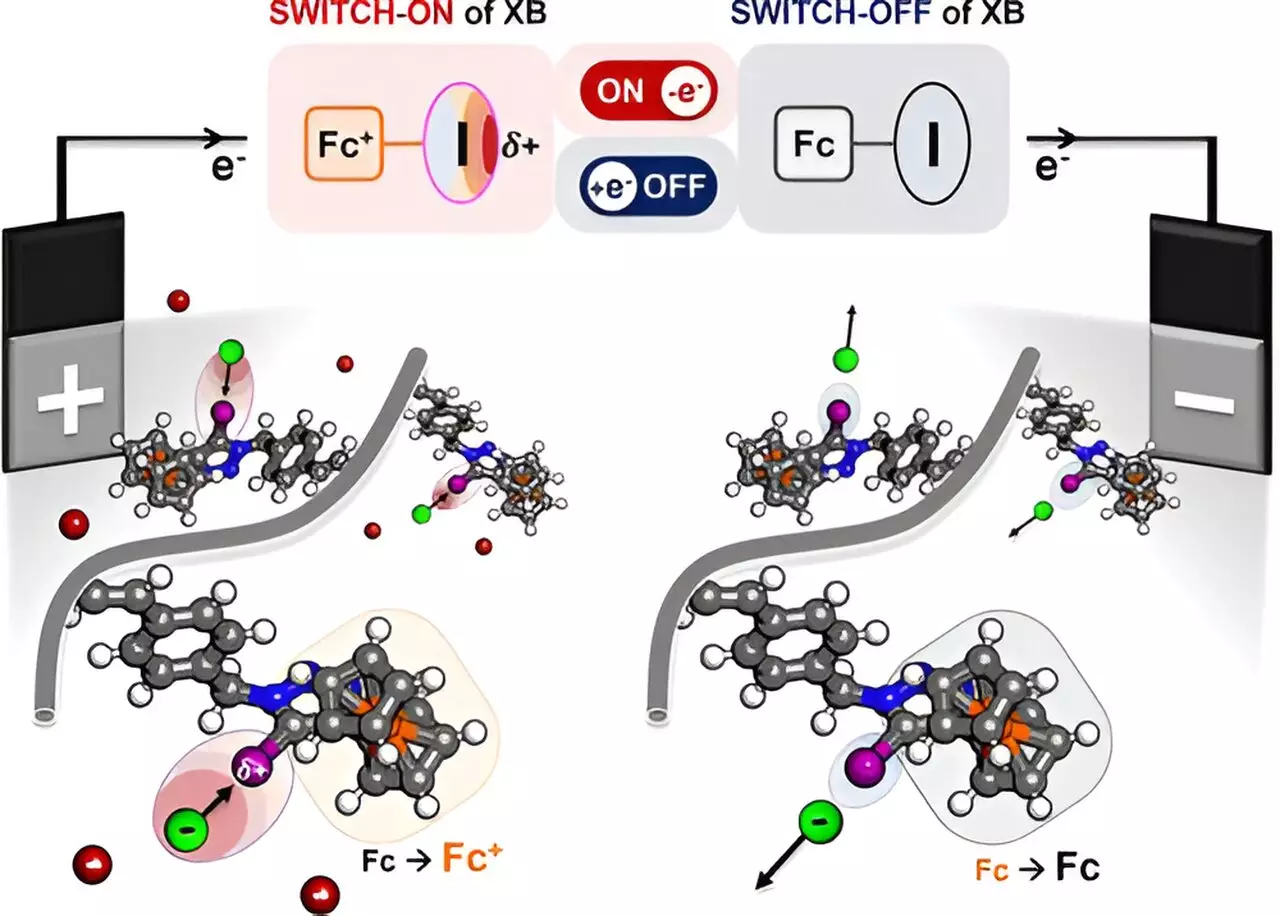
At the core of this technology lies an engaging scientific principle known as halogen bonding. The polymer engineered by Su’s team features a halogen atom—specifically iodine—coupled with ferrocene, which acts as a redox-active center. When electricity is applied, the molecular structure adjusts, activating the halogen’s “sigma hole.” This charge attracts negatively charged ions, enabling the polymer to selectively pull specific targets from a solution based on their unique interactions with the halogen.
Nayeong Kim, a graduate researcher integral to this project, emphasized the experimental nature of this approach to halogen bonding. By exploiting the inherent properties of halogen atoms, the research team has opened the doors for potential applications that were previously unexplored. The tunable nature of the polymer provides advantages over conventional methods, which often lack this selective focus.
The experimental results revealing the polymer’s selective properties were confirmed using techniques such as nuclear magnetic resonance (NMR) and Raman scattering. These validations act as a solid foundation for future studies and practical applications. The path ahead involves optimizing the polymer’s efficiency and exploring methods for scaling this technology to industrial levels.
Collaborative efforts with professor Alex Mironenko have allowed for computational modeling of the polymer, further illuminating the complex mechanisms activated upon the application of electricity. Together, the researchers are undertaking the challenge of enhancing the purity of isolated chemicals, vital for their application in various fields.
One of the most compelling advantages of this research is its potential environmental impact. By reducing the reliance on energy-intensive methods and minimizing waste, this electrochemical approach provides a more sustainable option for chemical separation. As industries increasingly face pressure to adopt greener practices, this “electric sponge” technology could play a crucial role in making chemical processes more eco-friendly.
This technology also aligns perfectly with the growing demand for specialized chemicals required in various applications, from drug synthesis to advanced materials production. As industries shift toward greener practices and more efficient methodologies, innovations like this polymer could prove essential.
The research from the University of Illinois Urbana-Champaign exemplifies a significant leap forward in the realm of chemical engineering. The introduction of an electrically activated polymer capable of selective separation synergizes scientific innovation with the pressing need for sustainability. As the field progresses, refining and scaling this technology could not only optimize chemical processes but also herald a new era of environmentally conscious industry practices. The electric sponge symbolizes a fusion of creativity and scientific rigor, promising to revolutionize how chemicals are separated and utilized in the future.