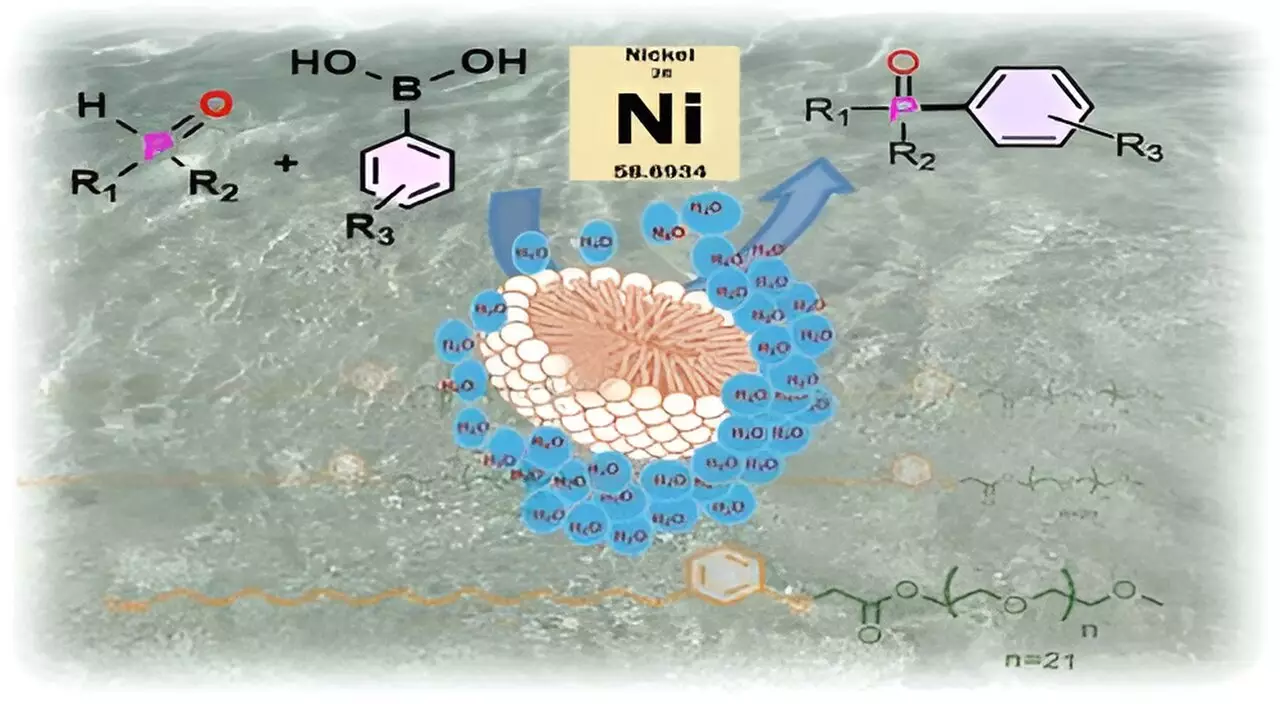
In the realm of chemical synthesis, particularly within industrial settings and laboratories, the majority of reactions occur in a liquid phase. This allows substrates to effectively intermingle, leading to the desired chemical transformations. However, the ubiquitous reliance on organic solvents introduces a significant drawback: many substrates, along with their catalysts, are often sensitive to moisture, leading to unwanted side reactions. Consequently, the chemical industry is responsible for generating over 80% of its waste from these hazardous organic solvents, raising severe environmental concerns regarding their disposal and potential toxicity.
Acknowledging these challenges, a team of researchers from the Department of Inorganic and Physical Chemistry at the Indian Institute of Science (IISc) has taken an innovative step towards an eco-friendlier approach. Their latest work, recently published in ACS Sustainable Chemistry & Engineering, details the creation of a surfactant derived from agricultural waste—specifically, the byproducts of cashew nut processing. This novel surfactant, dubbed CNSL-1000-M, leverages cashew nut shell liquid (CNSL), a sustainable resource given India’s position as the second-largest producer of cashews worldwide.
Driven by the principle that any substitute for organic solvents must originate from bio-based materials, the researchers designed CNSL-1000-M by effectively combining cardanol—a naturally hydrophobic compound extracted from CNSL—with m-PEG, a hydrophilic polymer. This synthesis creates surfactants that are not only environmentally friendly but also effective in facilitating chemical reactions in aqueous environments, thus potentially replacing hazardous organic solvents.
The innovation of CNSL-1000-M lies not only in its source but also in its function. When the surfactant is introduced to water, it self-assembles into micelles—tiny spherical structures with a unique makeup. The hydrophobic components orient themselves inward, creating a central pocket that is shielded from water. This configuration allows moisture-sensitive catalysts and substrates, such as nickel complexes, to reside in a protective environment, analogous to a football floating in a pond. Just as a sealed football remains dry inside, the micelle protects its contents from adverse reactions with water.
What’s especially compelling about micellar catalysis is its reflection of natural biochemical processes. Many enzymes in biological systems effectively utilize hydrophobic pockets to facilitate essential reactions. By mimicking this natural process, the CNSL-1000-M surfactant allows for the catalysis of critical chemical reactions, specifically in forming carbon-phosphorus bonds crucial for various industrial applications, including the development of pharmaceuticals and organic electronics.
The application of CNSL-1000-M signifies a leap forward in sustainable chemistry. The research highlighted that using this novel surfactant resulted in up to 80% higher yields in aqueous environments compared to traditional methods employing organic solvents. Even when pitted against existing surfactants, CNSL-1000-M achieved a remarkable 30% increase in product yields, showcasing its potential as a more efficient catalyst. Furthermore, the ability to substitute expensive catalysts like palladium with cost-effective nickel complexes marks another significant advantage, making processes more economically viable.
Additionally, the temperature reduction required for these reactions enhances energy efficiency, further contributing to a more sustainable model for chemical synthesis.
Looking ahead, the research team is eager to bridge the gap between the lab and industrial application. By collaborating with industry partners, they aim to facilitate a transition from hazardous organic solvent-based reactions to safer, greener aqueous micellar technologies. The vision is clear: a future where sustainable practices prevail in chemical production, harnessing the power of agricultural waste and environmental consciousness for a better tomorrow.
The innovative utilization of CNSL-1000-M surfactant exemplifies a critical shift towards sustainable organic synthesis, addressing the substantial challenges posed by traditional chemical methodologies. As the field evolves, the implications for industry, environment, and health are profound, potentially reshaping the landscape of chemical manufacturing for generations to come.