Recent advancements in the field of chemistry have unveiled significant breakthroughs concerning the oxidation potentials of positive ions. A research group led by Professor Ingo Krossing from the University of Freiburg has succeeded in pushing the boundaries of these potentials beyond the conventional limits. With a focus on improving the interaction dynamics between solvate ions and their solvent environments, this pioneering work heralds new opportunities for both academic research and practical applications in electrocatalysis.
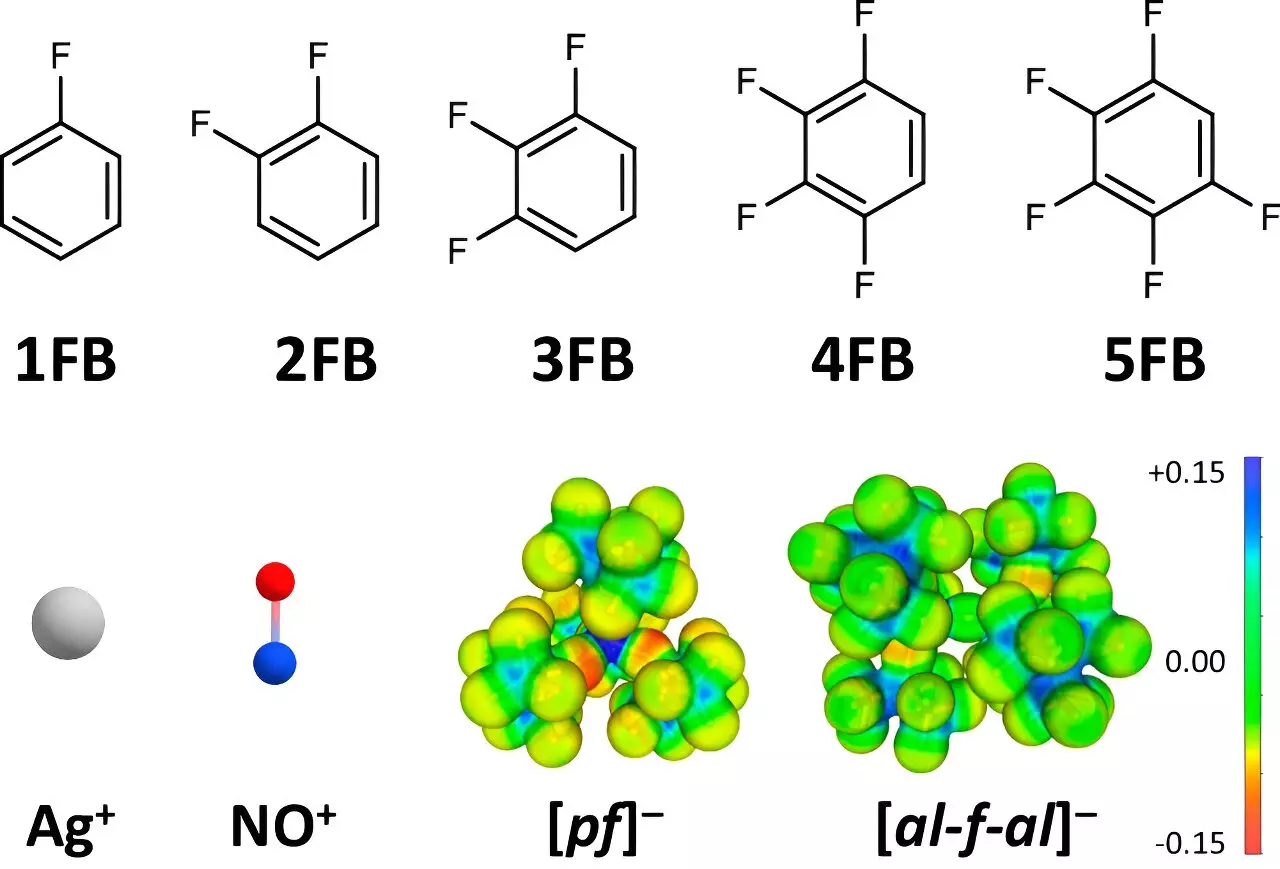
Traditionally, the oxidation potential of key positive ions, such as Ag+ and NO+, has been capped at values around +0.65 to +1.0 V vs. Fc+/0 when addressed in standard solvents. However, the Freiburg team has reported an extraordinary achievement by reaching potentials of +1.50 to +1.52 V vs. Fc+/0. This groundbreaking innovation hinges on the clever utilization of weakly coordinating solvents and anions, particularly fluorinated benzene derivatives, which possess unique structural properties that enable higher oxidation potentials.
The research employed a strategic approach by substituting polar groups to maximize the electronic characteristics of these fluorinated compounds. By carefully selecting solvents that interact minimally with the positive ions, the team was able to minimize deviation in oxidation potential caused by the solvent environment.
The effective oxidation abilities of small and highly charged positive ions like Ag+ and NO+ are generally compromised by their strong interactions with surrounding solvents and anions. The current study meticulously examined how different configurations of fluorinated benzene derivatives influence these interactions. In collaboration with Dr. Johannes Hunger from the Max Planck Institute for Polymer Research, the team scrutinized the dielectric constants of these solvents, revealing that fluorinated variants exhibited significantly higher dielectric values than traditional solvents like dichloromethane or acetone.
The research posits that with increasing fluorination, the interaction between positive ions and their surroundings is effectively reduced. Dr. Malte Sellin, a co-author of the study, noted that the two-fold to four-fold fluorinated aromatic compounds reduced interactions compared to their non-fluorinated counterparts. This reduction in interaction results in a less hindered environment, allowing for previously unattainable oxidation reactions.
The implications of these findings are enormous not only for the academic community but also for various sectors that rely on advanced redox chemistry. The significantly enhanced oxidation potentials open a new vista of possibilities for conducting redox reactions that were previously deemed impractical. Moreover, this innovative methodology could lead to the development of groundbreaking electrocatalysts and redox mediators that improve efficiency in various chemical processes.
The complex interplay between solvent chemistry and positive ion behavior is at the core of this research, setting the stage for a series of new elementary chemical studies. The research team’s successful demonstrations portray their findings as not just an exploratory endeavor but as an essential stepping stone towards practical applications in fields such as energy storage, catalysis, and material synthesis.
The concerted efforts of Professor Krossing and his team represent a significant milestone in advancing our understanding of positive ion chemistry. By mastering the delicate balance between solvation and ion interaction through weakly coordinating solvents, researchers have paved the way for novel applications that have the potential to revolutionize the field of electrocatalysis. As further research unfolds, these exciting developments could alter the landscape of material science, energy generation, and chemical manufacturing. With additional explorations anticipated to stem from this foundational work, the future of redox chemistry appears increasingly promising.