Microplastics have emerged as a pervasive environmental pollutant, present in myriad ecosystems across the globe. As bodies of water face seasonal changes, particularly during frigid months, an intriguing phenomenon occurs: tiny plastic particles undergo significant transformations when trapped in ice. Recent research from experts in environmental science has shed light on how freezing can alter the size, buoyancy, and overall impact of these microplastics, with implications for freshwater and marine ecosystems alike.
Microplastics, which are defined as plastic particles smaller than 5 millimeters, have diverse origins—from the degradation of larger plastic debris to the shedding of artificial fibers from clothing. Their presence has profound implications for aquatic life, posing ingestion risks to marine and freshwater organisms, which can lead to bioaccumulation of harmful substances in the food chain. However, one aspect that remains critical yet underexplored is how environmental factors like temperature changes influence microplastics’ behavior.
The recent study published in Environmental Science & Technology meticulously examines the behavior of various polymer types—namely polyethylene (PE), polyurethane (PU), and polytetrafluoroethylene (PTFE)—under freezing conditions. The authors hypothesized that the physical state of microplastics as affected by freezing would result in a spectrum of changes that could modify their environmental fate. Their findings reveal that several factors, including particle density, salinity, and clever scientific experimentation, contribute to a more nuanced understanding of how freezing microplastics can lead to a shift in their environmental consequences.
Chunjiang An and colleagues delved into intricate laboratory experiments, examining how freezing affects the size of microplastic particles. Notably, they found that fresh water seems to facilitate a noteworthy expansion of microplastic size when frozen. In the freshwater samples, PE particles exhibited a substantial size increase of about 46%, while PU particles only increased by 9%. This difference is premised on the hydrophilic properties of PU that facilitate water interaction, causing these particles to remain more dispersed in icy conditions.
A particularly significant observation was that under increased salinity, the size alterations post-freezing were negligible, pointing to the operational role of brine channels within the icy matrices. These channels serve as microscale havens, allowing microplastics to avoid merging into larger aggregates, thereby challenging previous assumptions that freezing uniformly influences particle size.
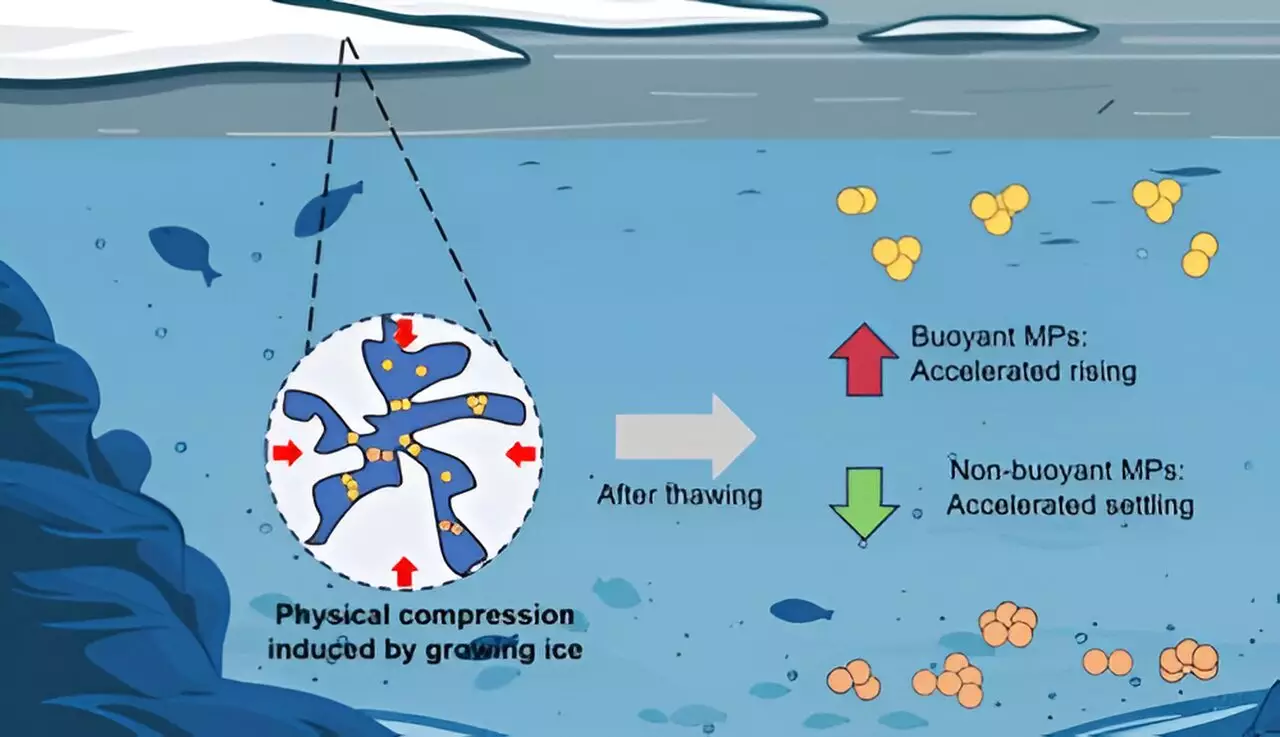
A critical takeaway from the study is the correlation between freezing and particle buoyancy. Prior to the freeze-thaw experiments, the behavioral dynamics of each polymer were predictable based on their density relative to water. However, after undergoing the freeze-thaw cycle, the particles subsequently displayed pronounced changes in their ability to float or sink. It was observed that PE particles, with lower density, experienced heightened buoyancy, allowing them to resurface more rapidly than before. In contrast, denser polymers like PTFE and PU tended to settle quicker, now impacted by an augmented gravitational force.
The researchers employed calculations of competing forces—gravity, buoyancy, and drag—to demonstrate that the alterations induced by freezing significantly affect microplastic sedimentation patterns. This suggests that seasonal freezing and thawing might dynamically impact the sedimentation processes of microplastics in natural water bodies, ultimately affecting their interaction with aquatic life and ecosystems.
Though the current study provides vital insights into the lifecycle of microplastics released from ice, it must be noted that the duration of freezing used in experimental settings was relatively brief compared to natural phenomena. The implications of prolonged periods of freezing remain largely unexplored but are crucial for accurate predictions regarding microplastic behavior in real-world scenarios.
As we continue to grapple with the growing challenge of plastic pollution, understanding how environmental conditions influence microplastics is imperative. Future research should expand on these findings, focusing on longer freezing durations and varying environmental contexts, to establish a comprehensive understanding of microplastics’ fate in our ecosystems. This foundational study serves as a call-to-action for scientists and environmentalists to develop strategic approaches that mitigate the ecological risks posed by these tiny yet impactful pollutants.