In a remarkable advancement, researchers at the Ulsan National Institute of Science and Technology (UNIST) have introduced a groundbreaking catalyst that mirrors the efficiency of natural enzymes in dismantling toxic hydrocarbons. Spearheaded by Professor Jaeheung Cho from the Department of Chemistry, this innovative development offers a promising avenue towards a more sustainable and eco-friendly method for pollution reduction. The details of this research, published in the Journal of the American Chemical Society on June 3, 2024, may very well signal a turning point in environmental chemistry.
The Mechanics Behind the Madness: Harnessing Nature’s Designs
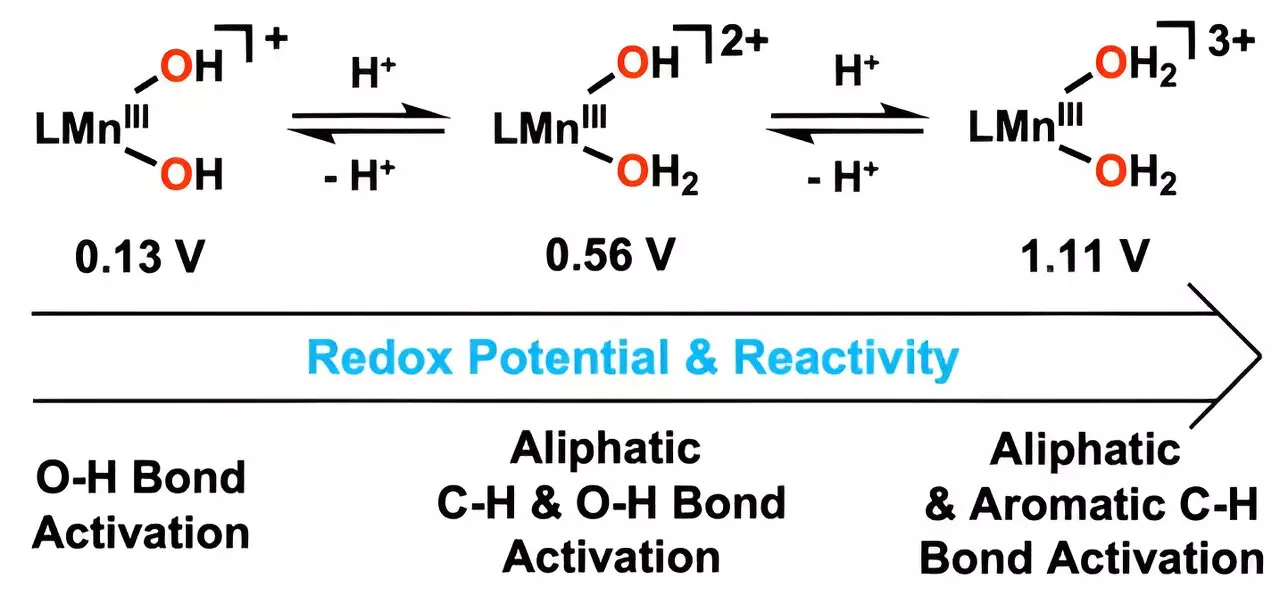
What differentiates this novel catalyst is its extraordinary ability to replicate the function of metalloenzymes found in nature. These enzymes have long been the biological models for chemical reactions, specifically for hydrocarbon oxidation. By mimicking these biological systems, the UNIST team was able to create a catalyst that can efficiently oxidize carbon-hydrogen (C-H) bonds at significantly lower temperatures compared to traditional approaches, thereby enhancing energy efficiency in chemical processes.
Through a meticulous synthesis process, the researchers integrated metal-bound water molecules with hydrogen ions into the catalyst design. This combination significantly improved the catalyst’s performance. In particular, the manganese catalyst showed a dramatic increase in reaction rates, leading to a faster breakdown of harmful molecules without the need for excessive energy input.
Durable Efficacy: From Theory to Practice
One notable experiment involved the oxidation of anthracene, an aromatic hydrocarbon notorious for its insolubility and chemical stability. Typically resistant to degradation, anthracene poses significant environmental challenges due to its toxicity. The ability of this new catalyst to effectively oxidize such stubborn compounds at lower temperatures illustrates its transformational potential in industrial applications.
With a focus on enhancing the reduction potential of manganese, the researchers managed to construct a structure capable of targeting strong C-H bonds. Professor Cho remarked on the unprecedented nature of this achievement, noting that it marks the first occasion where a manganese(III) complex, equipped with dual water molecules, effectively engaged with aromatic hydrocarbons. Such specificity and efficiency could pave the way for a reimagined approach toward managing hazardous waste.
The Industrial Implications: A Green Frontier
This breakthrough not only illuminates a path for cleaner industrial processes but also highlights the urgent need to innovate within the realm of environmental chemistry. As industries grapple with the demands of sustainability, the development of catalysts that reduce pollution through lower-energy methods could revolutionize standard practices. The implications here extend beyond mere scientific curiosity; they align closely with global efforts to promote greener technologies and the reduction of harmful emissions from fossil fuels.
The work undertaken at UNIST embodies a crucial intersection between natural systems and technological applications. As researchers continue to explore the depths of enzymatic functions, the fight against hydrocarbon pollution inches closer to fruition. The burgeoning field of catalysis promises not only to lessen our environmental footprint but also to serve as a beacon of hope in the battle against climate change. With each scientific advancement, we move a step closer to a sustainable future, and the UNIST team’s contributions may well be at the forefront of that change.