The journey of drug development is notoriously grueling, often spanning over a decade and costing billions of dollars before a product reaches the market. Astonishingly, around 90% of drug candidates falter during clinical trials, with many never even getting that far. These staggering failure rates can largely be attributed to safety concerns. The stakes are incredibly high; when a drug harms instead of heals, the consequences can be catastrophic—not only for patient health but also for pharmaceutical companies’ investments and reputations.
One key issue that has plagued the drug industry is the inherent inability to predict how prospective drugs will interact with biological systems. For years, researchers have relied on laboratory experiments to assess drug toxicity, but this approach is not only time-consuming but also expensive and often yields uncertain results. Essential breakthroughs in this arena are sorely needed, which is where cutting-edge technology comes into play.
The Role of AI Models in Drug Screening
Researchers at the Broad Institute of MIT and Harvard have embarked on a transformative venture by utilizing artificial intelligence to predict the biological impact of new drug candidates before they enter animal testing or human trials. Srijit Seal, a visiting scholar at the Carpenter-Singh Lab, has pioneered machine learning models designed to assess chemical and structural features indicative of toxicity. These models aim to offer an early warning system for drug developers, elucidating potential risks associated with drug candidates.
Seal’s innovations have led to the creation of various tools that estimate how drugs may affect critical parameters like cellular health, pharmacokinetics, and organ functionality—especially concerning the heart and liver. While the findings are preliminary, with papers already published in respected journals, they represent a significant step forward in how we can approach drug safety.
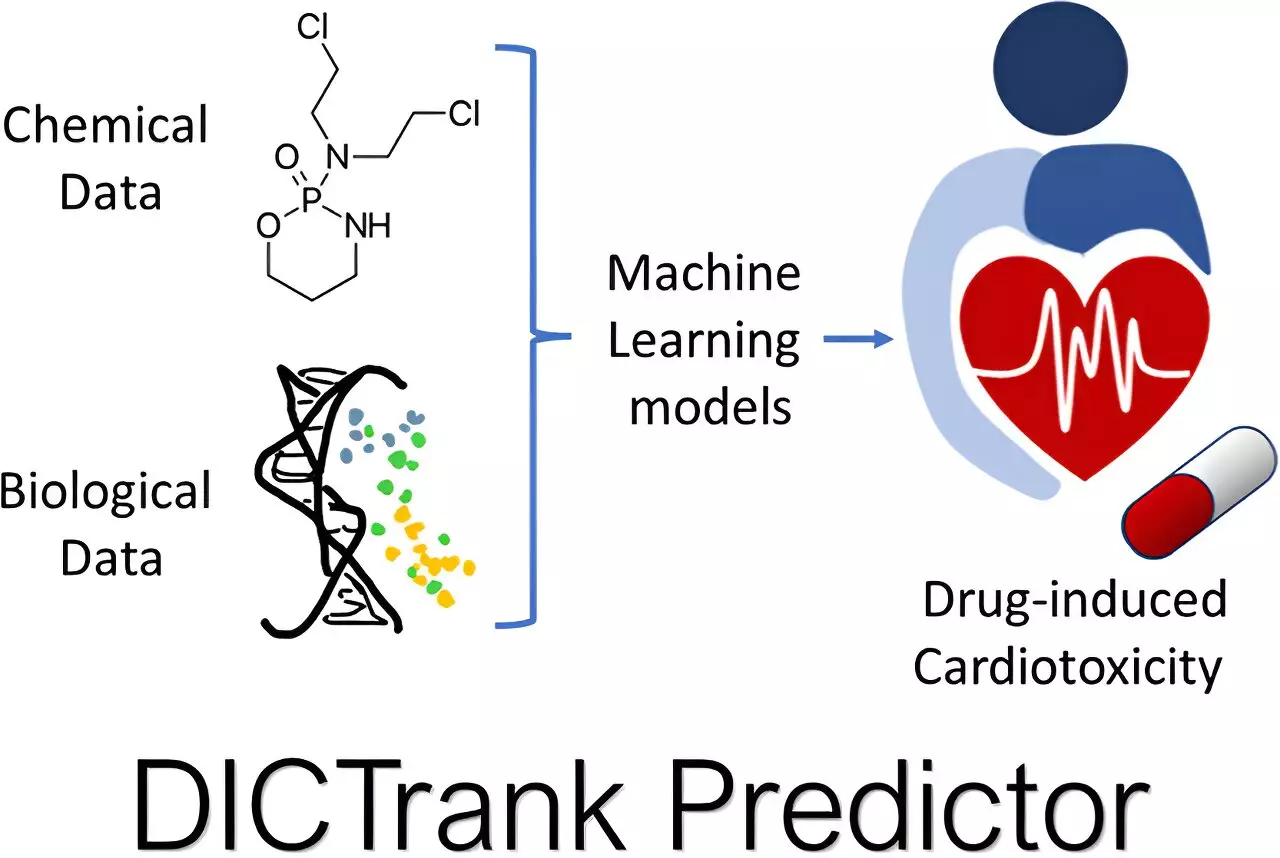
Precision in Toxicity Prediction
One of the core functionalities of these AI models is their capacity to predict different types of drug toxicity. For instance, the development of the DICTrank Predictor represents a groundbreaking effort to assess drug-induced cardiotoxicity (DICT) based on data curated by the FDA. The sheer ability of these models to draw connections between chemical structures and adverse effects not only streamlines research but also helps developers focus on the most promising candidates.
Additional challenges arise when extrapolating these findings from animals to humans. Seal and his team have tackled this by crafting the DILIPredictor, which successfully differentiates toxic reactions observed in animals from those that might occur in humans. This capacity to identify compounds that are non-toxic in humans, despite being toxic in animal models, offers a tantalizing glimpse of the future of targeted drug development.
Pharmacokinetics and the Future of Drug Efficacy
Understanding how a drug is absorbed, distributed, metabolized, and eliminated—collectively known as pharmacokinetics—is essential for determining its efficacy and safety. Yet, traditional pharmacokinetic modeling can be labor-intensive and resource-heavy. Seal’s initiatives in developing predictive models for pharmacokinetics offer a novel methodology for addressing these challenges.
By implementing machine learning techniques, Seal anticipates that researchers can identify potentially successful drug candidates more efficiently, effectively allowing them to “fail faster.” This approach enables the allocation of resources to the drugs with the highest likelihood of success, thereby optimizing the entire drug development process. Such a paradigm shift could drastically alter the landscape of pharmaceutical research, where time and efficiency equate to significant financial savings.
BioMorph: Bridging Data and Biological Understanding
Moreover, the quest for accuracy in understanding drug interactions goes beyond mere toxicity predictions. To provide more profound insights into how compounds affect cellular dynamics, Seal has developed BioMorph, an ingenious deep learning model that incorporates imaging data alongside metrics relating to cell health. By leveraging CellProfiler’s capabilities, BioMorph aligns traditional data with modern machine learning, creating a more nuanced interpretation of how a drug affects cells at the morphological level.
The implications of BioMorph extend into practical realms, offering scientists valuable context and understanding regarding the biological consequences of drug interactions. It demystifies the often complex changes occurring within cells due to various compounds, offering interpretative clarity that can guide further investigation.
The convergence of machine learning and drug development promises to enrich the pharmaceutical landscape dramatically. By providing predictive tools that focus on safety and efficacy, researchers not only have the potential to enhance the quality of new drug candidates but also to foster a more meticulous and scientifically sound approach to drug discovery. As technological advances continue to reshape this field, the implications for public health and medical innovation are burgeoning with potential.