The journey of gallium, a remarkable metal first discoverable in 1875 by French chemist Paul-Émile Lecoq de Boisbaudran, continues to astonish scientists and researchers even nearly 150 years later. Recent revelations from a team of scientists at the University of Auckland have shed light on its uncharted structural and behavioral characteristics, significantly altering our understanding of this intriguing element. Whereas traditional studies have largely accepted gallium’s unique qualities, current investigations have reached groundbreaking conclusions that challenge long-accepted paradigms regarding its atomic behavior and thermal properties.
Gallium’s notoriety stems from its fascinating physical characteristics—most notably its low melting point, making it notorious for dissolving a spoon made of it in a hot cup of tea. This peculiarity has not only captured public imagination but has also made gallium an essential component in modern technologies, particularly in semiconductors. The role of gallium in the evolving landscape of technology underscores its importance more than ever, calling for an in-depth exploration of its atomic foundations.
Dimers and Density: A Study of Uniqueness
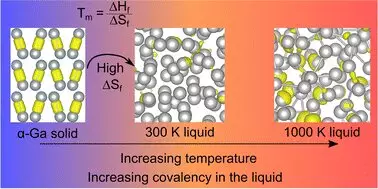
One of the most captivating aspects of gallium lies in its atomic structure. Unlike typical metals that exhibit a uniform density in both solid and liquid states, gallium exhibits a form known as ‘dimers’—pairs of atoms that share distinct covalent bonds. This uniqueness led researchers to uncover that gallium is actually less dense in its solid state compared to its liquid form, drawing an intriguing parallel to how ice floats on water. Herein lies the delicate dance between structure and behavior that makes gallium a subject of continued fascination.
In a recent study titled “Resolving Decades of Debate: The Surprising Role of High-Temperature Covalency in the Structure of Liquid Gallium,” published in *Materials Horizons*, scientists have challenged the long-standing belief that gallium’s covalent bonds vanish upon melting. Astonishingly, these bonds reportedly reappear at elevated temperatures, which compels researchers to rethink their prior assumptions regarding the properties of this metal. The implications of such a finding are profound—leading to a fresh narrative not just for gallium, but for our understanding of metallic behavior in general.
Entropy and the Mystery of Melting Points
The pivotal concept introduced by the researchers concerns entropy, which in the context of gallium, appears to govern the unique transition between solid and liquid states. The research proposes that the disappearance of covalent bonds corresponds with a substantial increase in entropy, effectively giving freedom to the gallium atoms, and thereby explaining its inexplicably low melting point. Such insights suggest a profound connection between thermal behavior and atomic relationships, pushing the boundaries of how chemists perceive metallic elements.
Professor Nicola Gaston, a key figure in this research, emphasizes that decades of literature have relied on fundamentally flawed assumptions regarding the structure of liquid gallium. This verdict not only addresses scientific inaccuracies but also ignites a spark for further exploration into the atomic behaviors of other metals that may have been similarly overlooked.
Applications in Technology and Beyond
The implications of enhancing our understanding of gallium craftsmanship extend far beyond theoretical realms; they reach into the practical world of technology and innovation. Gallium’s intrinsic properties are applied in numerous cutting-edge technologies, including semiconductors, photovoltaic devices, and even in areas like aerospace. Its application as a liquid metal catalyst in creating self-assembling structures could revolutionize material science, allowing for novel applications in nanotechnology and beyond.
Moreover, gallium’s presence in the evolution of telecommunications systems and high-performance computing solidifies its role as a cornerstone in modern engineering and scientific exploration. As this metal continues to be interwoven with advances in numerous fields, its application could even extend to astrobiology—offering a chemical fingerprint that aids scientists in searching for signs of past life on extraterrestrial bodies, such as Mars.
Redefining the Narrative of Discovery
The revelations surrounding gallium accentuate a broader theme often overlooked in the narrative of scientific discovery. The metal’s name pays homage to Gaul—reflecting its French roots—but its story is not limited to national identity; it represents a collective human endeavor to uncover meaning in the disorder of nature. The collaborative efforts at the University of Auckland demonstrate the power of scholarly pursuit and the persistent quest for knowledge that transcends geographical and temporal boundaries.
Gallium, though initially predicted before its discovery, has continually revealed secrets that challenge our comprehension of material science. As the study of this intriguing metal progresses, it invites other researchers to join in a collective effort towards unlocking the mysteries that nature still holds, reaffirming the notion that even established knowledge is ripe for reevaluation in the light of new evidence.