Aromaticity has been a cornerstone of organic chemistry for decades, primarily associated with compounds containing carbon. Traditionally, these compounds exhibit a unique stability and reactivity due to their electron-rich ring structures. They are often characterized by their distinct, pleasant scents, which is how they were originally identified. New research, however, beckons a reevaluation of this long-held doctrine. Recent breakthroughs, particularly the isolation of purely metal-based aromatic rings—specifically, those composed solely of bismuth—represent a paradigm shift in our understanding of aromatic compounds.
The Breakthrough: A New Kind of Aromatic Ring
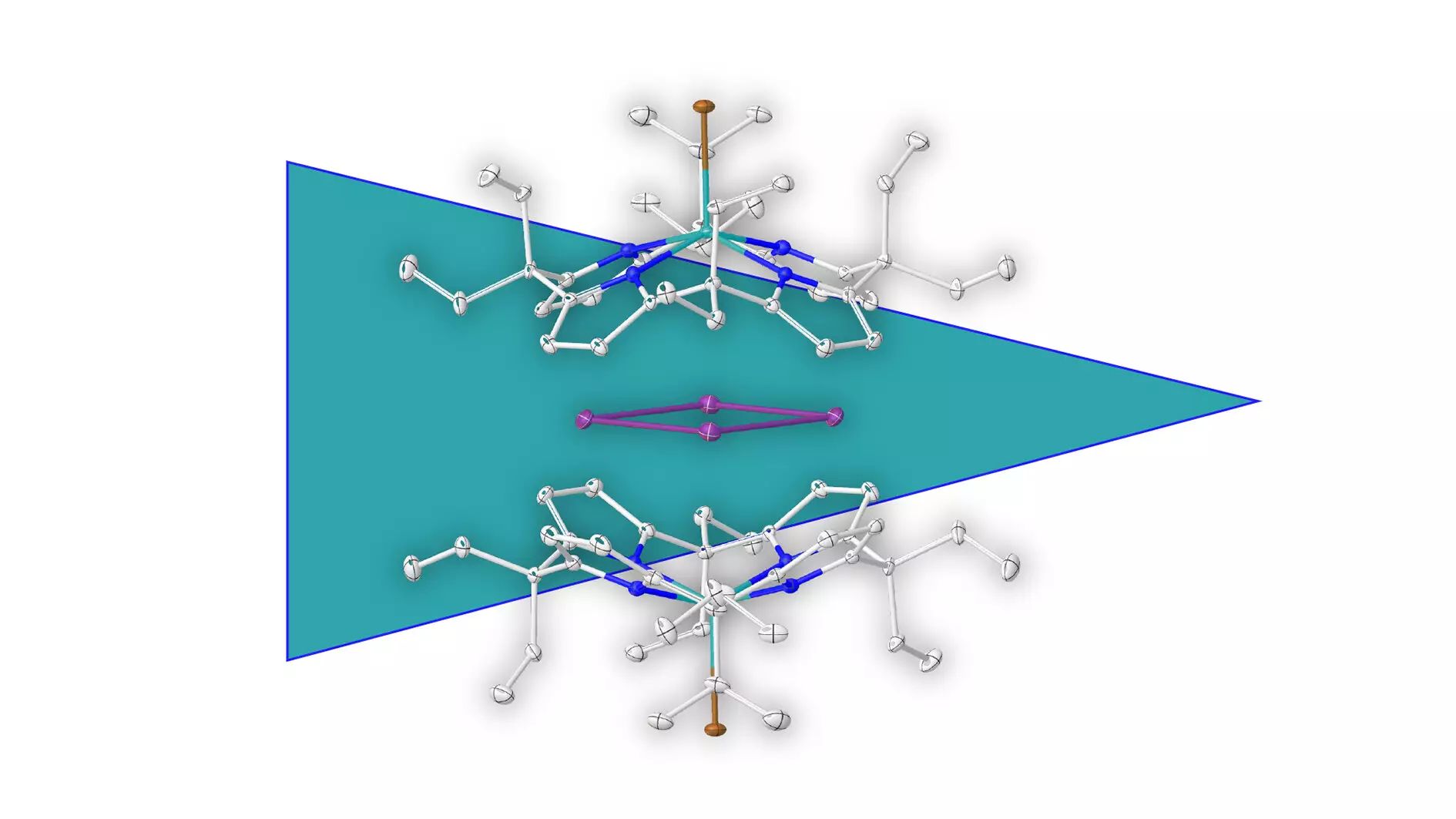
In a remarkable study led by Prof. Dr. Lutz Greb from Heidelberg University, the isolation of a metal ring that defies conventional classification has been demonstrated. Unlike traditional aromatic compounds that rely on carbon, this innovative work involves bismuth atoms forming a closed-loop structure. This discovery is not just an academic novelty; it has the potential to redefine our understanding of how metal atoms can interact in ways previously thought impossible.
The significance of isolating a metal aromatic ring lies not only in its novelty but also in the implications it carries for the field of chemistry as a whole. While we have known about metal complexes that bond with organic aromatic molecules, a ring composed entirely of metal defies the established definitions that have guided scientists for generations. This finding encourages the exploration of new classes of materials that may perform differently from traditional metal-organic hybrids.
Innovative Techniques in Stabilization
At the heart of this successful isolation is a revolutionary method of supramolecular stabilization. By encasing the positively charged bismuth ring in a negatively charged molecular shell, the research team effectively inhibited decomposition reactions that would normally render such an isolation impossible. This technique not only opens the door for further exploration into other metal-based aromatic structures but also speaks volumes about the creativity required to innovate in the domain of chemical research.
As Greb suggests, the methodologies employed in this study could lead to advancements in various fields of science, particularly those dealing with charge transport. By understanding how charged rings can be stabilized and manipulated, chemists may unlock new pathways for developing materials with unique electronic properties.
Implications for Future Research
This research is far from being just an isolated incident; it signifies a new chapter in the study of aromatic systems that could ripple across multiple domains of physical and material sciences. The potential for discovering other metals capable of forming stable aromatic rings could revolutionize how we approach electronic materials, catalysts, and even energy storage systems.
What we might be witnessing is not just an evolution in our understanding of aromaticity, but the birth of a new conceptual framework. As researchers continue to investigate these discoveries, we are likely to unveil a plethora of unexpected behaviors and properties associated with metal-based aromaticity. The implications extend beyond mere academic curiosity—the findings could lead to groundbreaking advancements in technology, paving the way for both theoretical and practical applications in modern chemistry.
As the scientific community digests these findings, one must reflect on the sheer ingenuity of this research. It exemplifies the very essence of what it means to push the boundaries of knowledge, encouraging future chemists to think outside the box in their own explorations.