Transforming waste into valuable products has long been a noble pursuit for chemists, with the dual aim of environmental sustainability and resource efficiency. The latest strides in this field showcase an intriguing blend of chemistry and renewable energy. An international consortium of scientists has embarked on an ambitious path to exploit electricity, specifically to convert carbon dioxide, a notorious greenhouse gas, into methanol—a promising liquid fuel. The implications of this research, recently published in *Nature Catalysis*, resonate deeply with both energy and environmental discourses.
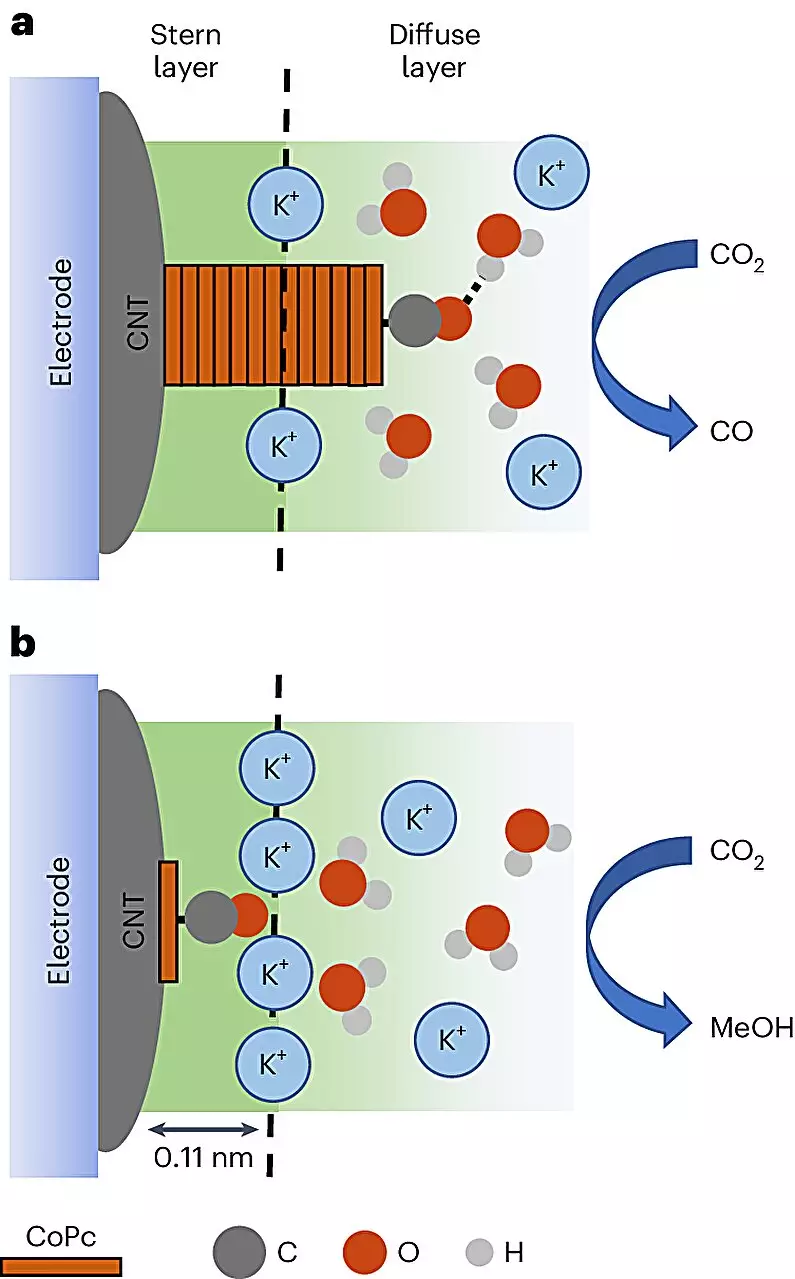
At the heart of the study is a pioneering approach that uses cobalt phthalocyanine (CoPc) molecules to catalyze the conversion of carbon dioxide into methanol while embedded in a sophisticated composite of carbon nanotubes. These tubes are renowned for their exceptional electrical properties and provide a novel platform to optimize chemical reactions through better material design.
Electrifying Catalysis: A Methodological Breakthrough
The researchers devised a unique method to apply an electrical current through an electrolyte solution that interacts with the CoPc molecules. This meticulously designed setup facilitates the transfer of electrons necessary for the conversion process. Intriguingly, this approach enables the real-time observation of catalytic reactions, which have traditionally eluded researchers. For the first time, the team could visualize how carbon dioxide molecules could navigate two potential pathways—either transforming into the desired methanol or an unwanted byproduct: carbon monoxide.
The critical insight from this research is that the likelihood of producing methanol depends heavily on the reaction environment. By adjusting the dispersion of CoPc on the carbon nanotube surface, the researchers were able to enhance methanol production up to eightfold. This indicates a pivotal advancement in catalysis that transcends traditional optimization methods, which often remain blind to the underlying mechanisms at play.
Innovative Insights through Spectroscopy
A significant aspect of the research involved employing an advanced form of vibrational spectroscopy. This cutting-edge technique provided a new lens through which the scientists could examine molecular behavior. The team, led by Quansong Zhu, demonstrated that they could differentiate between identical molecules in varying environments based on their vibrational signatures—a breakthrough that brings clarity to complex catalytic processes.
Such technological advancements are crucial for understanding not just how reactions happen, but also why certain catalysts excel over others. As Baker articulated, the knowledge gained here has the potential to catalyze further innovations across the field of chemistry, facilitating the development of catalysts that are both effective and efficient.
Cations: The Unsung Catalytic Heroes
Intriguingly, the research uncovered the role of supercharged particles known as cations in the methanol formation process. These charged particles appear to enhance the catalytic mechanisms in ways previously underexplored. This revelation opens a new avenue for further research, as understanding the nuances of cation interaction could pave the way for more effective catalytic strategies in various applications.
As this team uncovers the nuances of molecular interactions, the possibility of identifying new avenues for renewable energy sources becomes more pronounced. Rather than existing purely as a theoretical exercise, this work reveals a tangible, exciting potential for real-world applications that could reshape the future of energy production.
The Broader Implications of Methanol as an Alternative Fuel
Methanol is not merely a chemical curiosity—in many ways, it holds the keys to a greener future. With its high energy density and versatility, this liquid fuel can serve a variety of applications, from powering vehicles and airplanes to being harnessed for household heating and electricity generation. As we seek more sustainable alternatives to fossil fuels, methanol produced through renewable electricity emerges as a beacon of hope.
Baker’s insights into the broader impact of this research suggest that the findings may enable significant advancements in chemical discovery, as well as practical applications that extend beyond mere fuel. The exploration of methanol’s potential to become integral in energy production is compelling; it not only addresses the immediate concerns surrounding greenhouse gas emissions but also serves as a catalyst for economic activities centered around new energy technologies.
Through groundbreaking work and innovative methodologies, the path paved by this team distills the essence of scientific inquiry. Their research not only provides immediate benefits but also opens a door to numerous future possibilities in the realm of chemistry and energy. Harnessing the power of electricity to refine waste into usable fuels stands as a testament to human ingenuity and the transformative potential of science in addressing global challenges.