Cancer remains one of the most formidable challenges in modern medicine, and its complexity often hinges on the rapid proliferation of cancer cells. To tackle this issue effectively, scientists are increasingly turning their attention to the proteins that play pivotal roles in the survival and multiplication of these cells. Understanding these proteins—specifically, which parts of the proteins are essential—can pave the way for developing new cancer therapeutics. Traditional methods of protein profiling have, however, been inadequate. They often fail to reveal the full spectrum of potential protein targets, leading to missed opportunities in cancer treatment development.
Recent advancements made by a team of chemists at Scripps Research have shifted this paradigm by employing a dual-method strategy that aims to create a more comprehensive map of small-molecule-reactive proteins involved in cancer. By unveiling the intricate details of protein interactions, this research promises to enhance targeted therapies that may inhibit cancer cell growth more efficiently.
This innovative research harnesses the strength of two advanced forms of activity-based protein profiling (ABPP). The first method provides a broad overview of proteins interacting with various chemical compounds, while the second method zooms in on specific interaction sites between proteins and these compounds. According to co-senior author Dr. Benjamin Cravatt, this collaboration between methodologies allows for the identification of over 300 cancer-related proteins and their specific binding sites, fundamentally enhancing the specificity of potential drug targets.
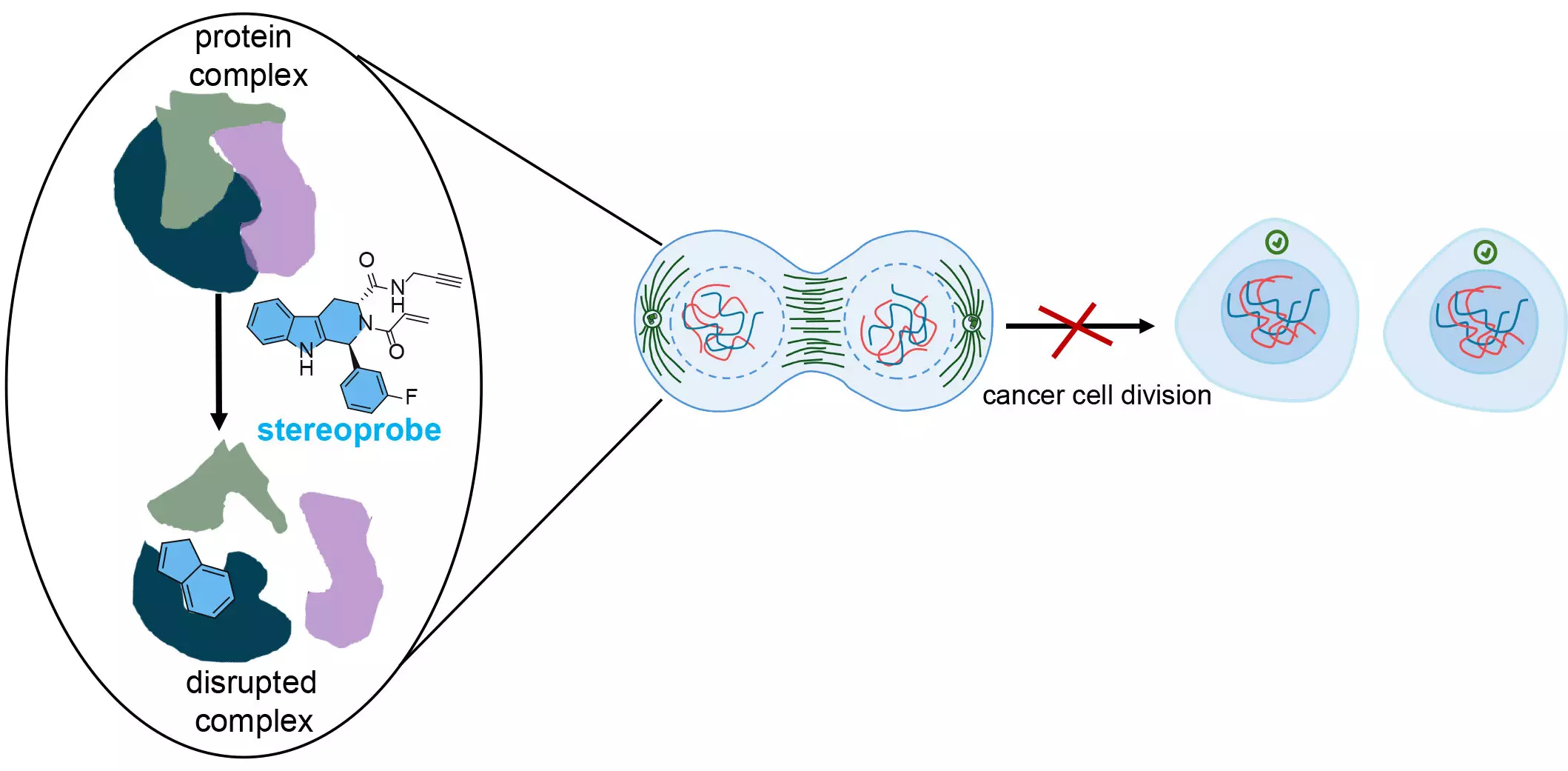
The researchers utilized a library of stereoprobes, which are specialized chemical compounds designed to bind permanently and selectively to proteins. Dr. Bruno Melillo, another co-senior author, elaborated on the unique design of these stereoprobes. A critical aspect of their design involved incorporating features that are often overlooked in conventional drug discovery, enhancing the likelihood of making novel biochemical discoveries that could translate into therapeutic advancements.
A focal point of this research is the amino acid cysteine, which is prevalent in many proteins, especially those associated with cancer. The researchers designed electrophilic stereoprobes that target cysteine, as they can create irreversible bonds that disrupt critical structural functions within proteins. This disruption can hinder cancer progression by impeding cellular mechanisms tied to cell growth and replication.
First author Dr. Evert Njomen highlighted that cysteine’s unique properties as the “most nucleophilic amino acid” make it a prime target for these chemical probes. By leveraging protein-directed ABPP and cysteine-directed ABPP, the team meticulously identified not only which proteins interacted with the stereoprobes but also the precise binding locations on those proteins.
The dual approach not only revealed extensive protein-reactivity data but also highlighted the inadequacies of relying on single-modality methods. The researchers noted that employing both techniques led to uncovering more potential targets simultaneously, providing richer data to guide therapeutic development. The findings confirmed that substantial information regarding protein interactions was lost when a singular methodology was utilized—a key insight that underscores the need for multifaceted approaches in cancer research.
This comprehensive profiling equips researchers with the tools to potentially develop drugs that specifically target these critical protein sites, thereby inhibiting processes vital to cancer cell survival. By strategically disrupting these functions, new treatments could significantly slow down or halt cell division. As Dr. Njomen notes, this state of “arrested development” could render cancer cells vulnerable to the immune system, which may lead to their elimination.
While the immediate focus is on developing targeted cancer therapies, the implications of this research extend far beyond oncology. Dr. Njomen expressed aspirations to formulate new libraries of stereoprobes geared toward addressing other diseases, such as inflammatory conditions. The principle of targeting proteins implicated in various pathologies holds promise across a spectrum of health issues, presenting an opportunity for profound advancements within the biomedical field.
As researchers continue to build on their new findings, the hope is that this dual-method approach will become a standard in drug target identification—leading to innovative treatments that could radically change the landscape of disease management and improve overall patient outcomes. The journey toward more refined cancer therapies is only beginning, but with significant strides being made in protein mapping, the future looks promising.