Polypropylene is an integral part of the modern manufacturing landscape, widely used in everything from packaging and textiles to medical supplies. As global awareness regarding sustainability escalates, the demand for this versatile polymer will likely continue to grow. At the heart of polypropylene production lies its primary precursor, propylene, which is traditionally derived from propane. This process has garnered significant attention due to not only its industrial feasibility but also its considerable environmental impact. Recent research has brought to light a groundbreaking, more energy-efficient method for producing propylene, raising hopes for a greener future in the industry.
Revolutionary Research from Leading Laboratories
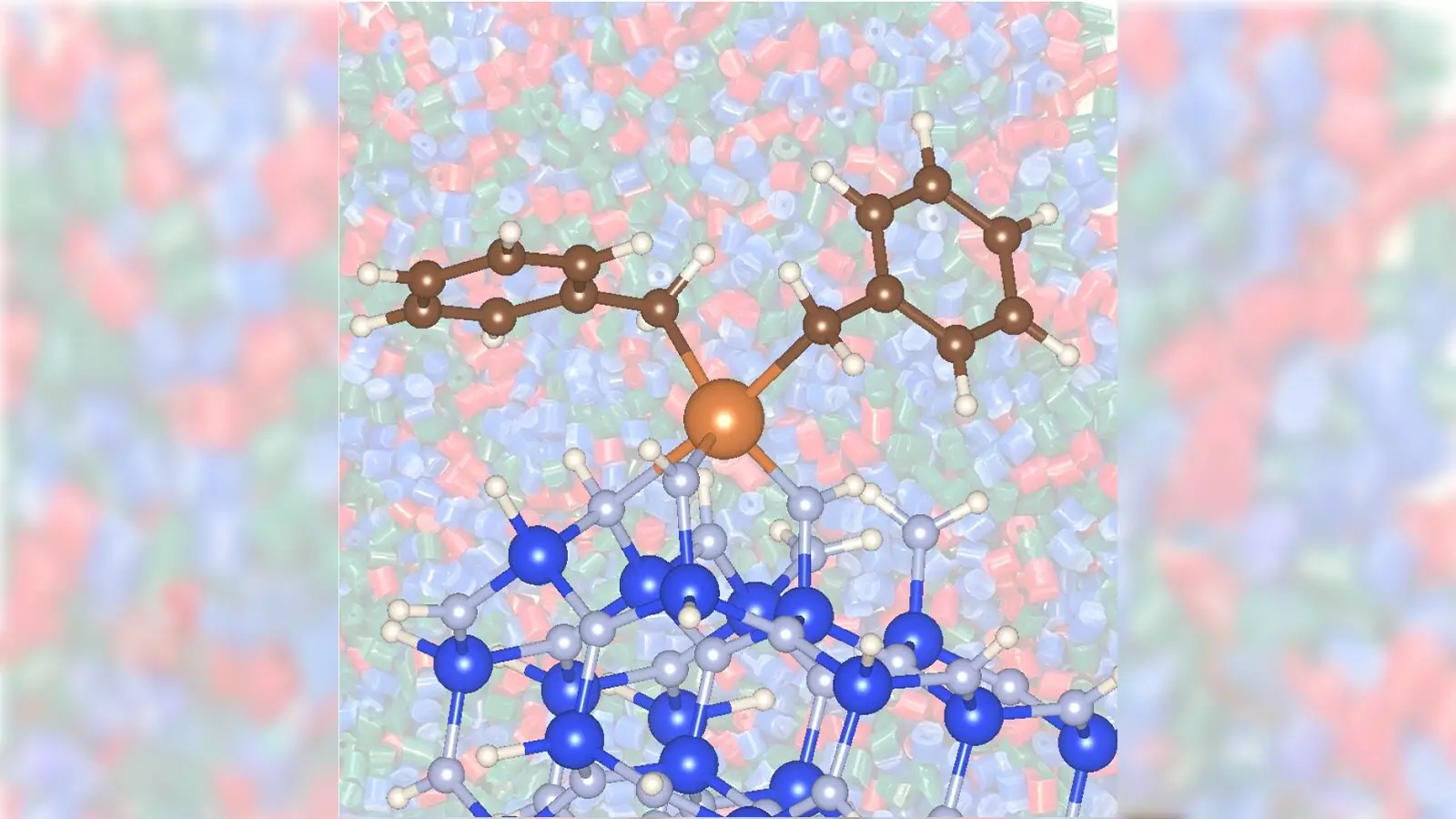
Recent findings from scientists at the U.S. Department of Energy’s Argonne and Ames National Laboratories have unveiled a novel approach that dramatically improves the catalytic conversion of propane into propylene using a zirconium-silicon nitride combination. This development marks a significant advancement over traditional methods which predominantly employ costly precious metals or less effective nonprecious catalysts, such as chromium. By swapping out these outdated materials in favor of less toxic and more affordable alternatives, the research holds the potential for not only boosting production efficiency but also mitigating environmental harm.
Enhanced Catalysis through Innovative Materials
A defining aspect of this research is the utilization of silicon nitride as a catalyst support. Traditional catalysts often require high operational temperatures, approximately 1,022 degrees Fahrenheit, which not only escalates energy consumption but also heightens carbon dioxide emissions—a significant contributor to climate change. The integration of zirconium with silicon nitride yielded promising results, achieving effective catalytic conversion at a lower temperature of 842 degrees. This indicates a leap forward in catalysis, indicating a path to greener production methods.
Moreover, the study demonstrated that silicon nitride supports increased catalytic activity, far surpassing the capabilities of silica supports. The implications of this are vast: less energy consumption translates directly into both economic and environmental benefits, making the entire production process more sustainable.
Broader Implications for Transition Metals in Catalysis
The implications of these findings extend beyond the scope of this particular application; they open a window into the possibilities of utilizing a wider array of transition metals in catalysis. The researchers have indicated that the unique properties of silicon nitride can enhance the catalytic activity of other metals, potentially changing the way we approach catalysts altogether. This line of inquiry represents an exciting frontier, offering a novel perspective on material reactivity in chemical processes.
The collaboration between different laboratories facilitated a comprehensive exploration that incorporated advanced imaging techniques, such as X-ray absorption spectroscopy and dynamic nuclear polarization-enhanced nuclear magnetic resonance. These innovative approaches have unveiled crucial insights into how the zirconium and silicon nitride interact, further reinforcing the effectiveness of this new catalytic system.
The Team Behind the Breakthrough
The success of this research is attributed not just to the innovative materials used but also to the collaborative spirit exhibited by the research team. Lead researchers David Kaphan and Max Delferro, along with an array of other partners, leveraged their collective expertise to push the boundaries of traditional catalytic research. Their shared insights and findings underscore the importance of teamwork in scientific discovery, reinforcing that groundbreaking advancements often emerge from the synergy of diverse ideas and specialized knowledge.
The journey of this research illustrates the inherent potential locked within underutilized materials and untried methods, suggesting a path forward filled with promise. This isn’t merely an incremental upgrade; it represents a paradigm shift that could revolutionize how we produce essential chemicals needed for everyday products.
Looking Toward a Sustainable Future
With this newfound understanding of catalytic processes, the future of propylene production appears increasingly promising. As demand for polypropylene continues to accelerate due to its ubiquitous applications, the transition to sustainable methods becomes not just an option, but a necessity. This research stands at the forefront of that transition, promoting innovations that are not only more efficient but also environmentally conscious.
Sustainability should be a guiding principle for industries moving forward, and the insights gleaned from this study provide a compelling blueprint for a more sustainable chemical production landscape. Investing in continued research along these lines could yield further advancements that contribute to reducing our carbon footprint and enhancing global efforts in combating climate change. The newfound efficiency and efficacy in propylene production signify much more than just scientific progress; they represent a step toward a more sustainable world where essential chemicals can be produced with a lower environmental impact. With ongoing research and dedication, the science of catalysis can indeed pave the way for a greener, more sustainable future.