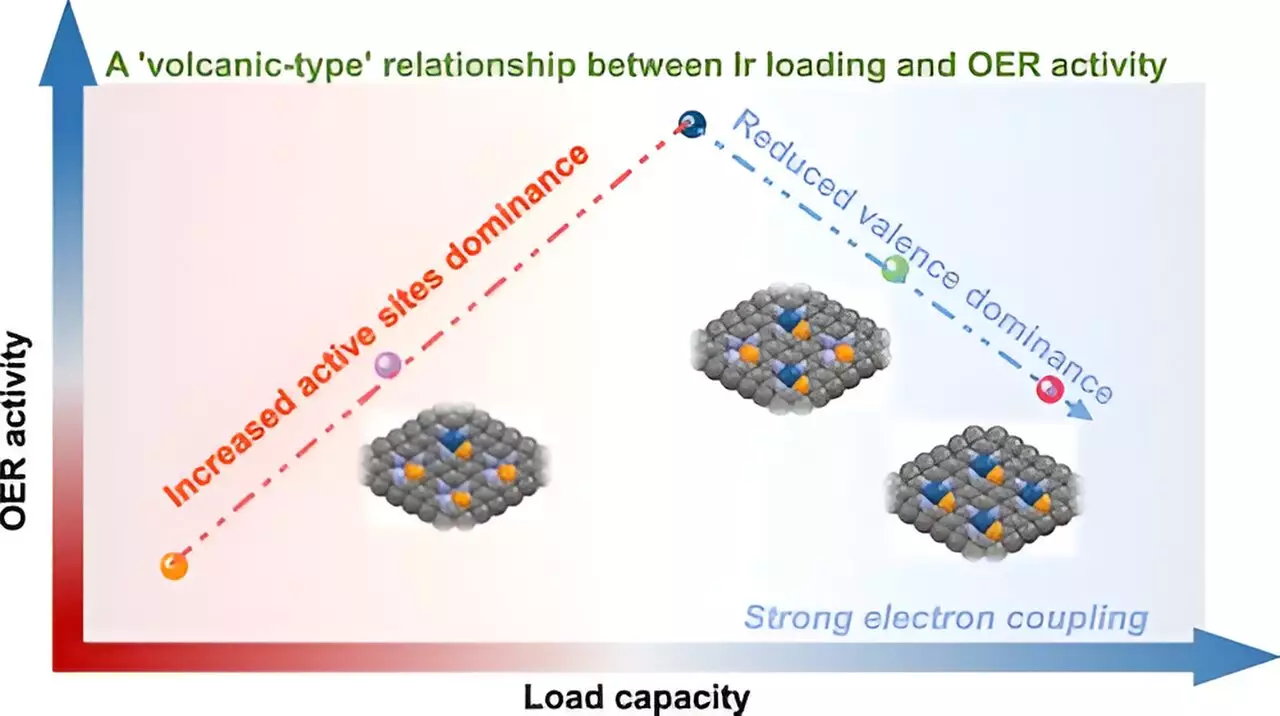
Recent advances in single-atom catalysts (SACs) herald new possibilities in efficiency and performance for catalytic reactions. A pivotal study led by Prof. Yan Wensheng at the University of Science and Technology of China (USTC) dives into the complexities of SACs, particularly focusing on the “volcano-type” relationship between metal loading and catalytic activity for the oxygen evolution reaction (OER). This concept challenges the established paradigms of how metal loading in catalysts directly correlates with their effectiveness, marking a significant shift in our understanding of single-atom catalysis.
The endeavor to create catalysts that maximize metal loading—while maintaining uniform atomic dispersion—remains one of the most pressing challenges in the field of catalysis. Most conventional approaches have struggled to find an equilibrium between high metal concentration and optimal catalytic performance. The work done by Wensheng’s team highlights that not all increases in metal loading lead to proportional gains in catalytic activity; instead, it reveals a more nuanced relationship that could redefine how researchers approach catalyst design.
The Significance of the Volcano-Type Relationship
The research illustrates an essential paradox: as the loading of iridium (Ir) increases, the number of active sites grows, initially enhancing catalytic performance. However, this relationship changes as iridium surpasses a critical threshold; the interaction between neighboring Ir atoms intensifies, altering their electronic characteristics and diminishing overall effectiveness. This insight is crucial, as it uncovers a tipping point where the dynamics shift from synergy to competition among active sites, ultimately revealing the fragile balance within SACs.
Utilizing a straightforward P-anchoring strategy, the team synthesized various Ir single-atom catalysts (x-IrPN3@C)within a metal loading range of 5% to 21%. Through advanced techniques like synchrotron radiation X-ray Absorption Spectroscopy (XAS), they unraveled the stabilizing role of the Ir-P coordination structure. This architectural arrangement of atoms is key to preventing Ir aggregation at high concentrations, thus maintaining optimal catalytic properties.
Innovative Methodologies and Insights
The study utilized an array of analytical techniques, including X-ray photoelectron spectroscopy (XPS) and theoretical calculations, to thoroughly investigate the underlying principles governing the observed volcano-type relationship. By understanding how valence states and local electronic environments adapt with varying metal loadings, researchers can refine their strategies toward designing more effective catalysts. For instance, the implications extend beyond iridium; this concept could be applied to other metals within the catalyst space, thereby influencing a broader set of reactions and applications in catalysis.
In essence, this groundbreaking research by Wensheng and his team provides a clearer framework for understanding how metal interactions at the microscopic level manifest at the macroscopic scale of catalytic behavior. By grasping the delicate interplay between metal loading and catalytic activity, researchers can make informed decisions aimed at optimizing catalyst design, ultimately promising more economical and efficient catalytic solutions across a range of industrial applications.
As we stand on the edge of a new era in catalysis, it is apparent that exploring this volcano-type behavior could lead to exciting developments in sustainable energy production and beyond, paving the way for an innovative leap in the mechanisms that drive our reactions at the atomic level.