As the world grapples with pollution, micropollutants have emerged as ghostly adversaries that infiltrate even the purest water sources. These trace chemicals, often from pesticides, pharmaceuticals, and industrial activities, are present in alarming quantities and can pose significant health risks. Traditional methods of removing these pollutants are often inefficient, leading researchers to explore innovative approaches that might turn the tide. One such frontier is photocatalysis, a process that harnesses sunlight through advanced materials to break down toxins. Yet, the complexity of these minuscule enemies requires equally sophisticated tactics, prompting scientists to delve deeper into the mechanics of photocatalytic reactions.
Unveiling the Power of Photocatalysis
Photocatalysis employs semiconducting materials like titanium dioxide (TiO2) under sunlight, intending to catalyze chemical reactions that mitigate pollution. While TiO2 has been a workhorse in this realm, recent advancements bring gold nanoparticles into the fray as co-catalysts. This marriage of materials has ignited enthusiasm among researchers, prompting investigations into how these nanoparticles can enhance the efficiency of pollutant adsorption on TiO2 surfaces. Specifically, the synergy between titanium dioxide and gold not only bolsters effectiveness in breaking down harmful agents but pushes the boundaries of how we perceive material interactions at the nanoscale.
The Breakthrough Imaging Technique
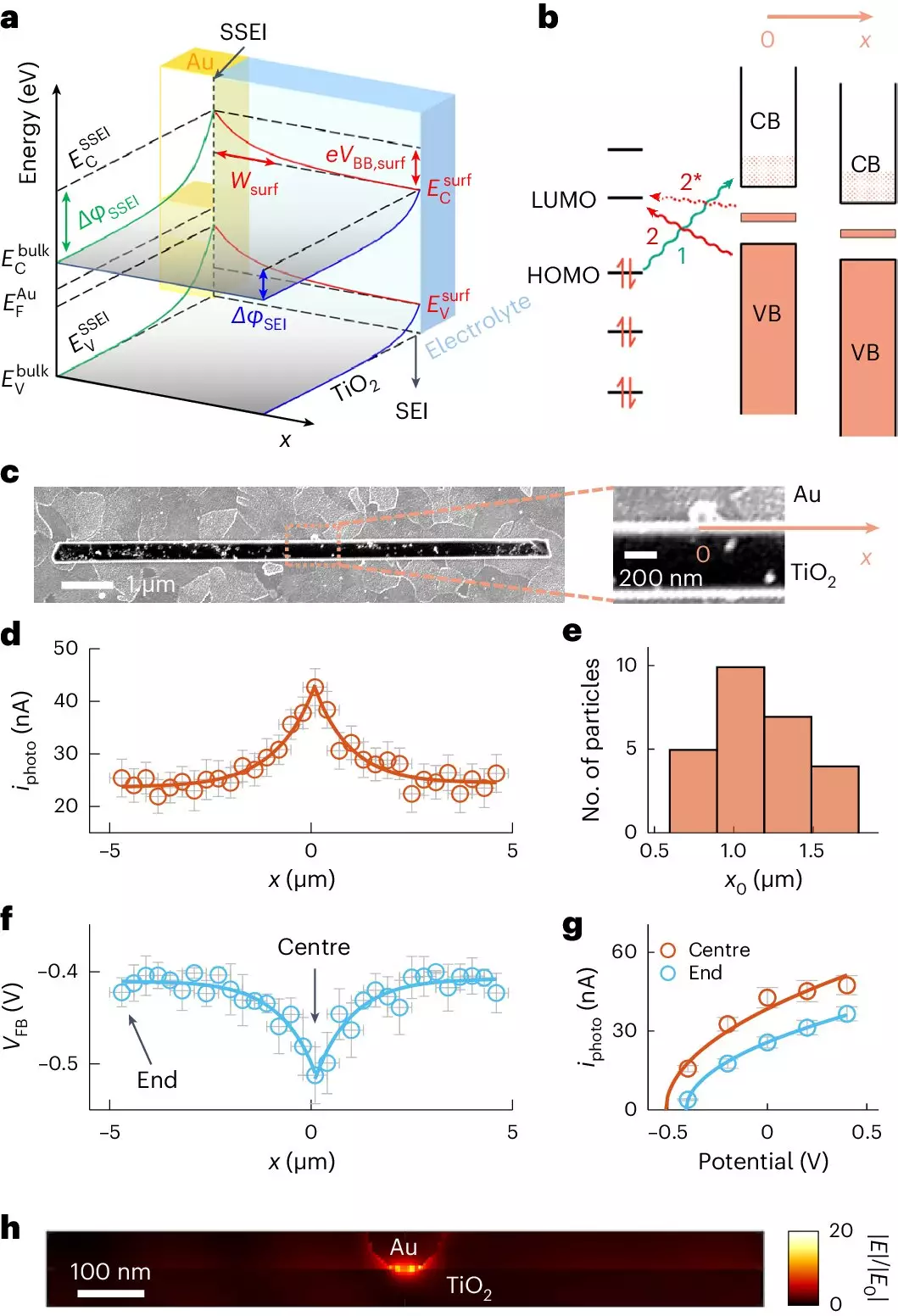
A remarkable stride in understanding this synergy comes from a research team at Cornell University, led by the eminent Professor Peng Chen. They developed a novel imaging technique called adCOMPEITS (Adsorption-based COMPetition Enabled Imaging Technique with Super-resolution). This groundbreaking method supersedes previous imaging modalities, delving into how molecules cling to the surfaces of semiconductors. In a fascinating twist, the researchers employed a fluorescent probe to visualize and compete with nonfluorescent micropollutants for adsorption sites on TiO2 surfaces.
By mapping these interactions with unprecedented precision, the team uncovered something remarkable: the introduction of a gold nanoparticle, just 100 nanometers in size, significantly increased adsorption efficiency across distances much greater than anticipated—up to ten times farther. This nuanced understanding opens up new avenues for enhancing photocatalytic processes and could lead to transformative changes in wastewater management practices.
Understanding Surface Band Bending
The linchpin of this discovery lies in a phenomenon known as surface band bending. When gold nanoparticles are incorporated into the TiO2 structure, they alter the electronic properties throughout the material’s surface. This alteration not only affects the immediate vicinity of the nanoparticle but extends its influence, creating a range of enhanced adsorption capabilities. The researchers found that the changes induced by the gold particles manifest exponentially, reaching micrometer distances from the original location. This newfound understanding sheds light on a previously elusive aspect of photocatalytic efficiency, suggesting that leveraging nanoparticle-enhanced adsorption could be a game-changer in environmental cleanup efforts.
Broader Implications for Environmental Science
What does this mean for the future of water treatment? The implications are vast and exciting. With a relatively small quantity of gold having such a marked effect on titanium dioxide’s adsorption capacity, the potential for improving photocatalysis becomes evident. This innovation could redefine the standards for energy-efficient pollutant removal, especially as the world shifts towards more sustainable practices. Moreover, the technique has applicability beyond mere pollutant degradation; it could significantly influence fields such as sensors and dye-sensitized solar cells, expanding its impact on technology and environmental science alike.
Not merely a triumph in academic research, the synergy between gold and titanium dioxide represents a pivotal step toward addressing the pressing issue of micropollution. As researchers continue to unveil the complexities of material interactions at the nanoscale, it is clear that the evolution of photocatalytic processes holds the promise of transformative change in the battle against environmental contamination. Embracing this knowledge could usher in a new era of effective, efficient, and sustainable solutions to one of the most urgent challenges facing our planet today.