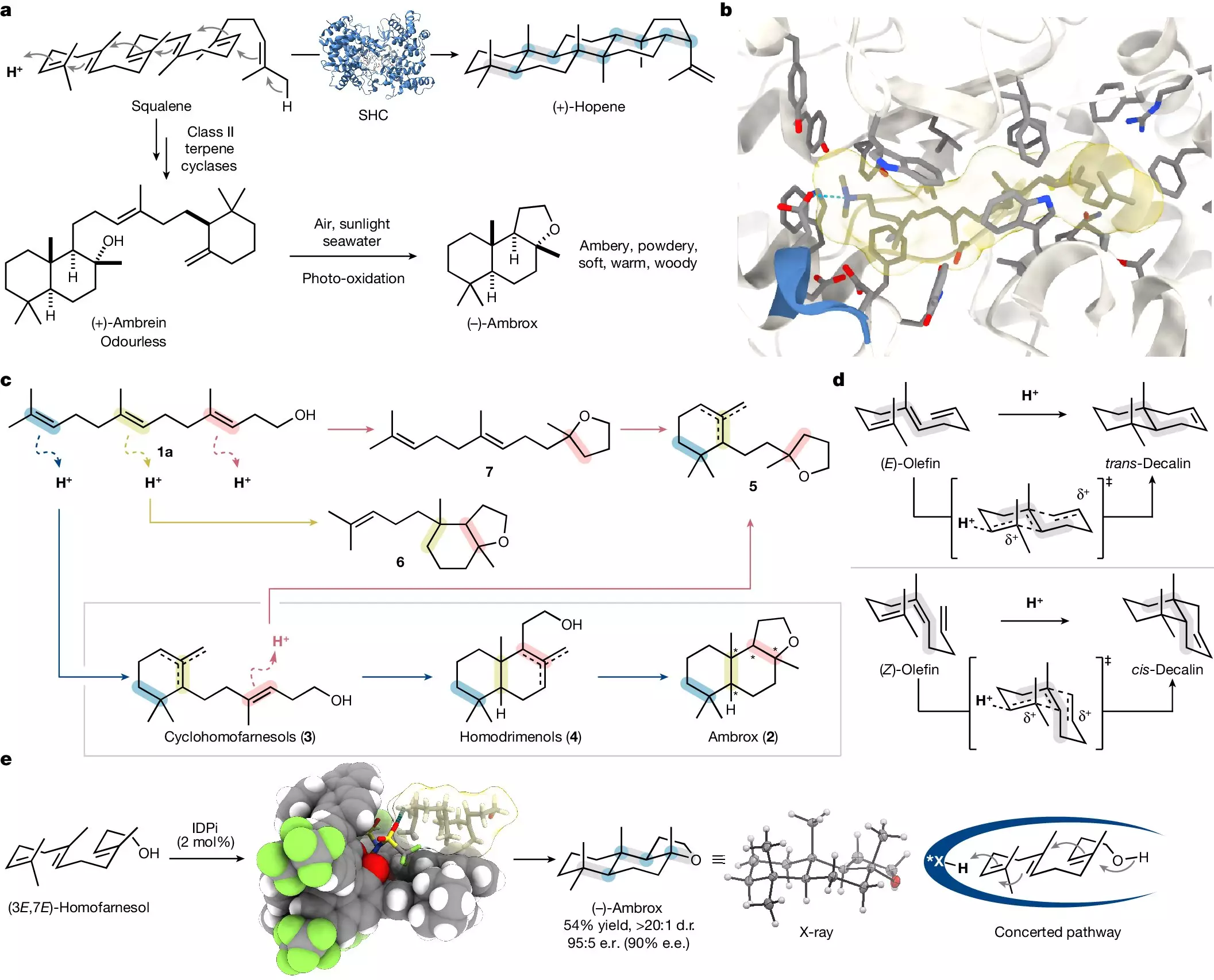
Fragrance has been a fundamental aspect of human culture for millennia, representing not only personal hygiene but also social status, health, and even spirituality. From the incense used in ancient rituals to the elaborate scents worn by nobility, mankind’s preference for pleasant smells has inspired innumerable innovations in perfumery. Among the most coveted scents in modern perfumery is ambrox, a complex compound traditionally harvested from ambergris—a rare waxy substance found in the digestive tract of sperm whales. Although this method provided ambrox to perfumers for centuries, the ethical implications and sustainability concerns have pushed scientists to seek alternatives.
Ambrox epitomizes the intricacies involved in the synthesis of fragrance compounds. The molecule has a chiral nature, which means that it exists in multiple mirror-image forms, but only one version delivers the ideal aromatic experience. Specifically, of the sixteen possible isomers, the desired sensory profile is achieved with just one. This enigma has confounded chemists for years, as creating such a specific compound not only requires precise techniques but also a sustainable source for the raw materials. Historically, the focus on ambergris raised significant ecological concerns, making the search for alternative synthesis routes an urgent priority.
Recent advancements in organic chemistry have heralded a new epoch for ambrox production. Led by Prof. Benjamin List from the Max Planck Institute for Coal Research, researchers have succeeded in synthesizing this famed scent from plant-based precursors. Essential to this breakthrough is the C15 molecule nerolidol, which can be derived from various plant sources in significant quantities. The collaboration with the chemical company BASF allowed for the conversion of nerolidol to a C16 building block called homofarnesol, which serves as the substrate for the final transformation into ambrox.
The innovative aspect of their approach is the use of a confined acidic catalyst in tandem with a specialized fluorinated solvent. This sophisticated setup allows for the selective synthesis of the desired isomer while maintaining mild reaction conditions. According to Dr. Na Luo, one of the paper’s lead authors, their method has not only made the reaction faster—producing ambrox in just one night instead of the typical three to four days—but has also ensured high selectivity.
One of the most compelling aspects of this research is the way it mimics natural processes. Polyene cyclization, the mechanism that unfolds to create structures like ambrox, is akin to how nature organizes molecular building blocks using enzymes. The elegance of this natural “toolkit” has inspired chemists for decades, and the realization of such complex reactions in the lab opens up new horizons for organic synthesis.
The confined catalyst employed by the List group acts as a pre-organizer, optimally positioning the substrate for transformation, while the fluorinated solvent provides stability to reactive intermediates. Together, these components allow the synthesis to be both highly selective and efficient, embodying the perfect balance of creativity and precision that characterizes great scientific endeavors.
The implications of this new synthesis method extend beyond mere academic curiosity. The ability to produce ambrox on a scalable level means that perfumers can access this prized compound ethically and sustainably. The List group’s process not only conserves resources—allowing both the catalyst and solvent to be recycled—but also represents a significant step toward greener chemistry practices. By using renewable starting materials and minimizing waste, this method promises to align fragrance production with modern sustainability goals.
The endeavor to synthesize ambrox inside a laboratory encapsulates a remarkable confluence of historical reverence for fragrances and cutting-edge scientific innovation. As the world moves toward sustainable practices, the work spearheaded by Prof. List and his team exemplifies how ingenuity can turn the tides in an industry long reliant on nature’s whimsical offerings. This research marks a significant milestone, opening a pathway for future explorations into complex organic compounds and their applications in perfumery and beyond.