Between the crystalline elegance of ice and the flowing nature of liquid water lies a fascinating relationship that has long puzzled scientists. Every day, we interact with this duality, whether it’s sliding on an icy sidewalk or savoring an ice cream cone on a hot day. Understanding this relationship is more than just a scientific curiosity; it is crucial for numerous practical applications, from climate modeling to the preservation of ecological balance. A recent study led by researchers from Kobe University and the Institute for Molecular Science has made groundbreaking strides in our comprehension of this interface, revealing insights that could reshape our understanding of the fundamental interactions between these two states of matter.
Revolutionary Techniques: Illuminating the Unseen
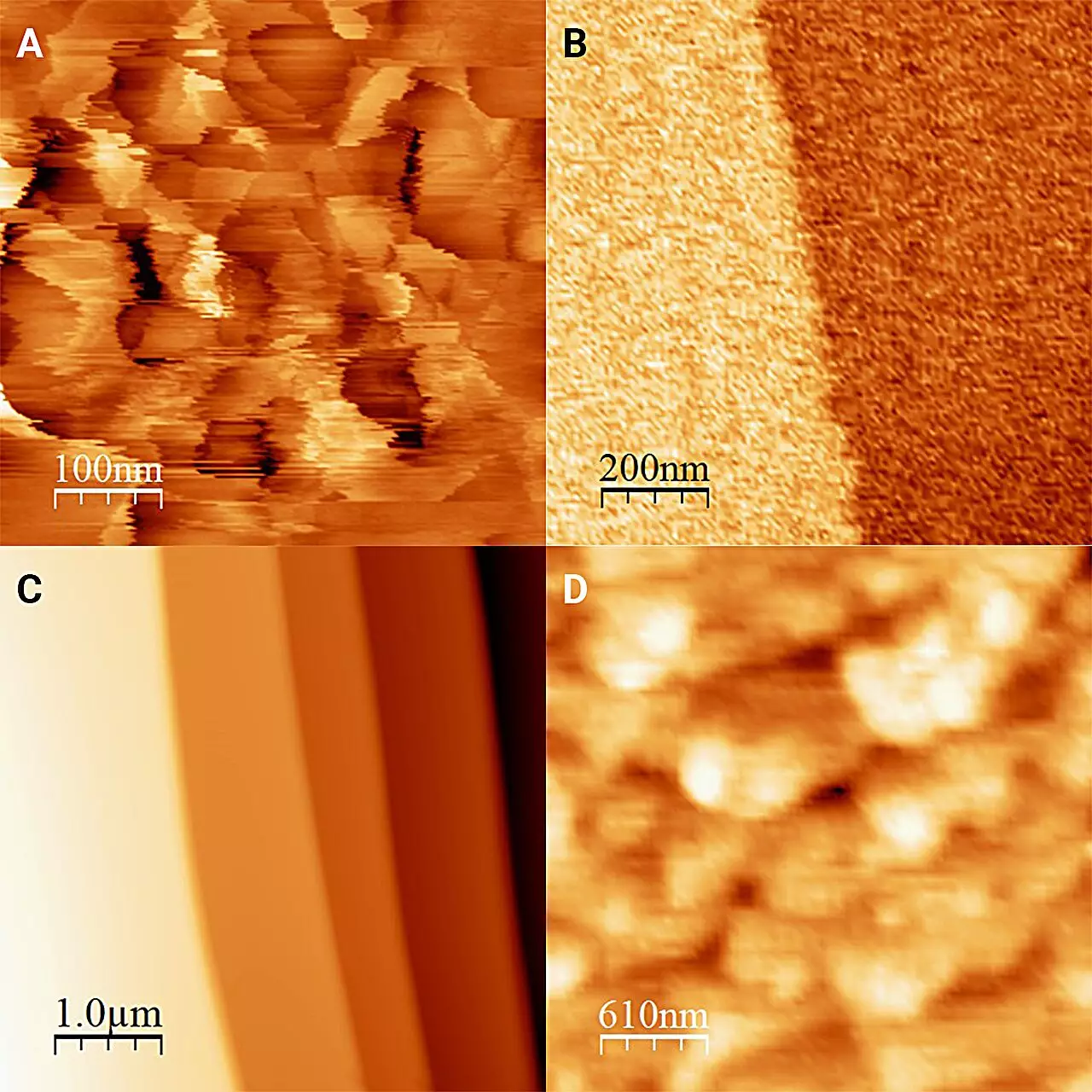
Historically, the investigation of the interface between ice and water has been fraught with challenges. The rapid transition from ice to liquid makes it exceedingly difficult to capture a static snapshot of their interaction. However, the innovative approach taken by the Kobe University team marks a significant shift in methodology. By immersing their observations in antifreeze—a liquid maintained below zero degrees Celsius—the researchers effectively minimized the melting of ice and could stabilize their readings at the critical interface. This novel application of refrigeration technology in microscopy not only highlights the creative problem-solving ability of the researchers but also illustrates the depth of resources available in modern scientific exploration.
The team faced numerous hurdles, including the complexities of maintaining stable equipment at sub-zero temperatures. This painstaking process showcases the meticulous nature of scientific inquiry, underlining the importance of perseverance and ingenuity. Indeed, the development of techniques such as this is not only crucial for studying ice but could have broader implications across various fields of science where temperature control is paramount.
Findings and Their Implications
The study’s results, published in The Journal of Chemical Physics, unveiled significant differences in the structure of ice when viewed through the lens of liquid interactions. The precision measurements demonstrated that ice in antifreeze appeared geometrically different from ice in the absence of liquid, defying earlier assumptions that ice remains unchanged in various aqueous environments. The flat, polished appearance of ice submerged in 1-octanol—an alcohol serving as antifreeze—contrasts sharply with the crystalline frost pillars observed in purely solid ice. Such observations prompt intriguing questions regarding the nature of crystallization and how environmental factors can alter the properties of solid materials.
Moreover, the researchers highlighted the hardness of this newly observed ice surface, revealing it to be substantially harder than previously understood. This newfound understanding could hold implications for fields as diverse as glaciology, materials science, and even culinary arts, where the characteristics of ice affect everything from the integrity of ice sculptures to the texture of frozen desserts.
Expanding Horizons: Future Directions for Research
Understanding the ice-liquid interface is just the beginning for this team of researchers. With their ambitious goals of enhancing microscopy resolution to the level of individual water molecules, one cannot help but feel a sense of excitement for what lies ahead. Such advancements have the potential to unlock a multitude of mysteries surrounding molecular interactions that govern not only ice and water but also a variety of other systems within the physical sciences.
By venturing into the realm of varying liquid conditions, the researchers have opened up new avenues for exploration. The study of different alcohols, which all presented unique altered ice features despite their similar properties, suggests a rich diversity of interactions waiting to be uncovered. Each finding propels the quest for knowledge further, hinting at complex underlying mechanisms that influence the molecular architecture of ice in natural settings.
In sum, the work of the Kobe University research team not only sets the stage for future advancements in understanding ice and liquid interactions but highlights the broader implications of such studies in our everyday lives. The tangible results of their investigation serve as a reminder of the intricacies of our natural world—a world that, upon deeper examination, reveals itself to be both strangely complex and beautifully interconnected.