Throughout history, the drive to innovate has been characterized by relentless experimentation and persistent refinement. Thomas Edison’s quest to discover the perfect filament for the incandescent lightbulb exemplifies this pursuit. After testing an array of materials, he finally perfected the tungsten filament, demonstrating that exploration and failure are fundamental components of scientific progress. Today, this approach is alive and well in contemporary engineering, especially in the realm of electrochemical energy storage. As society increasingly relies on advanced battery systems, understanding the materials and their behaviors has become paramount to fulfilling our energy needs effectively.
In modern laboratories, engineers and researchers regularly engage in systematic experimentation, but they also emphasize a deeper understanding of the principles governing material performance. Critical to this innovation are insights into how the components behave under various conditions. A recent study published in the *Proceedings of the National Academy of Sciences* unwrapped new knowledge concerning slurries—fluid mixtures essential in electrochemical applications such as batteries. This collaborative effort involved the University of Delaware, Northwestern University, and industry researchers, showcasing how interdisciplinary approaches can yield significant breakthroughs.
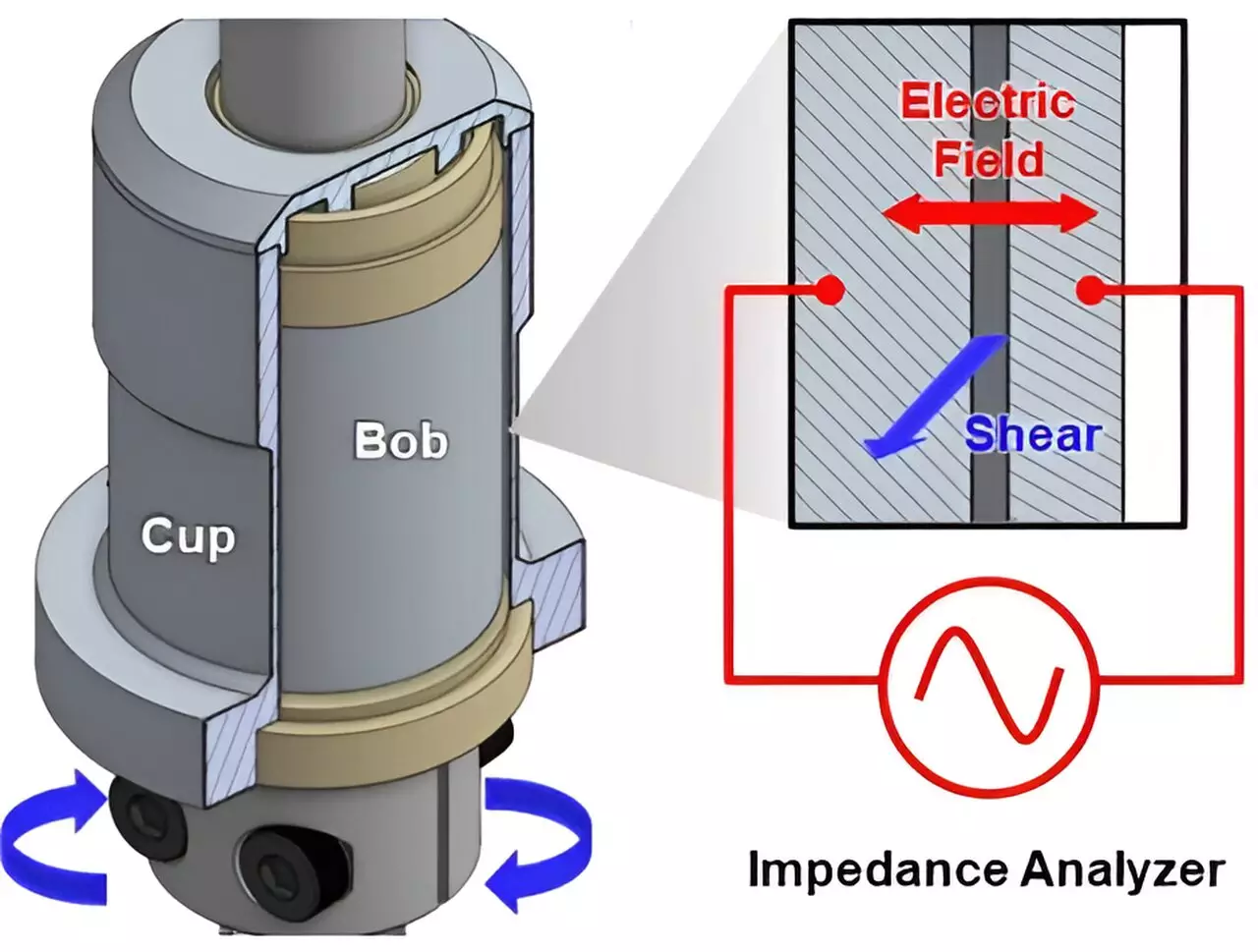
Central to the study was the exploration of electron mobility within conductive slurries, composed of particles suspended in a liquid medium. The research, led by Norman Wagner at UD and Jeffrey Richards at Northwestern, illuminated how electrons navigate through these complex materials—a crucial factor in determining the efficiency of devices like batteries. The authors emphasized that understanding the microstructure of slurries is just as essential as mastering their chemistry. Electrons need to ‘hop’ between conductive clusters suspended in a fluid, a process crucial for generating power.
To illustrate, envision racecars on a track. While every vehicle is equipped with fundamental components, such as engines and tires, the distinct assembly and design of each car significantly affect performance on the racetrack. Analogously, the makeup of battery materials—specifically how they are processed and assembled—can determine their functionality. Wagner aptly remarked, “To control device performance, it’s not enough just to control the chemistry; we have to control the microstructure, too.”
A prime ingredient in many electrochemical devices, including batteries, is carbon black, a conductive form of carbon composed of nano-sized crystalline aggregates. When incorporated into a slurry, these particles establish a conductive network allowing for electron mobility. However, because the particles are not rigidly connected, the efficacy of electron transport relies heavily on the slurries’ composition and manufacturing processes. Wagner noted that advancing our understanding of how carbon black behaves in these settings can lead to more efficient energy storage systems.
Prior studies by this research team utilized neutron-scattering techniques to delve into the behavior of carbon black in slurries, revealing that its rheological properties significantly influence the performance of electrochemical devices. The current study expands on these findings, offering a universal framework to understand the interplay between slurry composition and conductivity. This foundational work promises to provide practical guidelines for optimizing the design and manufacturing of electrochemical systems.
The implications of this research extend beyond mere academic interest; they pave the way for advances in various emerging technologies, particularly those involved in energy storage and conversion. One notable application lies in the development of electrolyzers, devices that generate hydrogen and oxygen from water through an electrochemical reaction. By leveraging insights from the study, improvements can be made to the material composition and processing techniques of these technologies, thereby enhancing their efficiency in producing renewable hydrogen for energy use.
As Wagner aptly summarized, “You can get the chemistry right, but if you don’t process it right, you don’t end up with the performance that you want.” This statement highlights a key challenge in the field: while understanding the chemistry of materials is crucial, processing methods must equally be addressed to achieve optimal results.
The relationship between material science and energy production is becoming increasingly complex and multifaceted. The collaborative effort between leading academic institutions and industry experts underscores the importance of a cohesive approach to innovation. By embracing both the chemistry and microstructure of materials, researchers can configure solutions that not only meet existing energy demands but also push the boundaries of what is possible. This ongoing commitment to experimentation and understanding will undoubtedly light the path toward a more sustainable and efficient energy future, illuminating the intricate web of science behind the devices that power our lives.