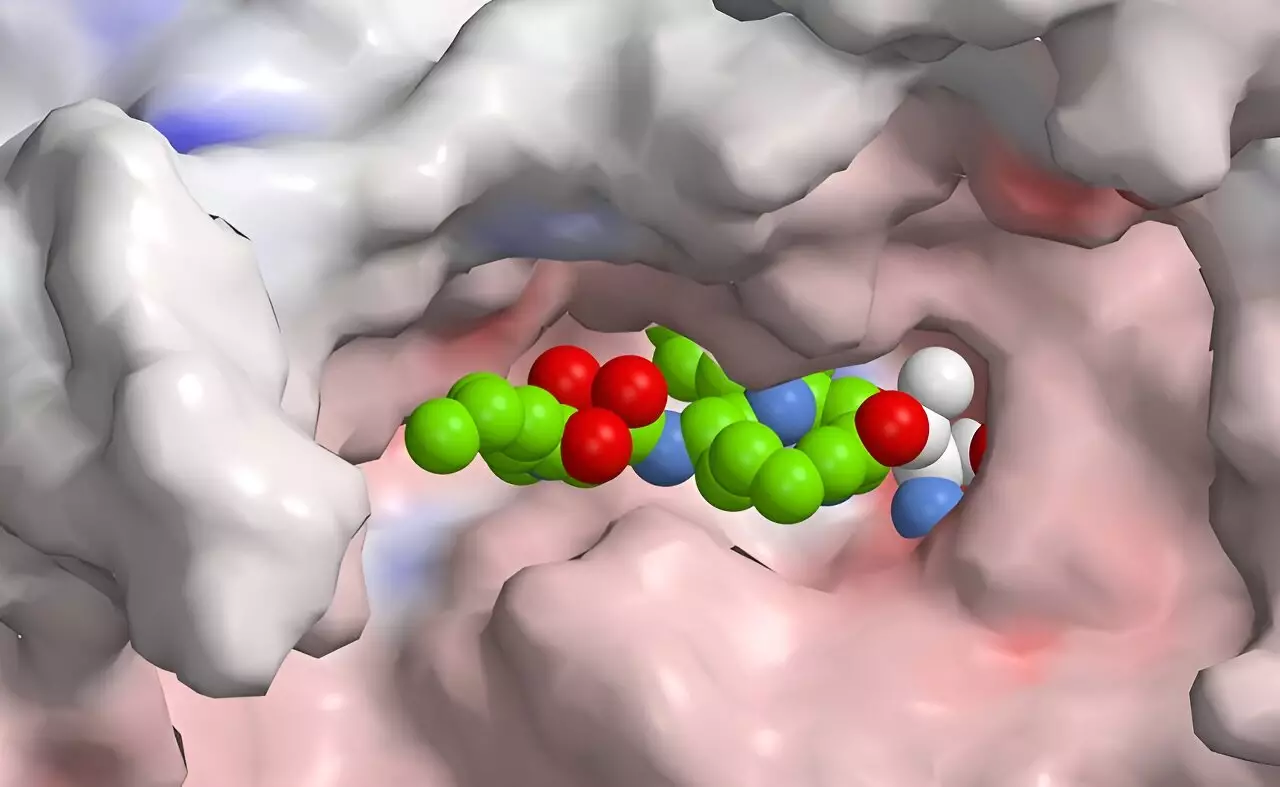
The immunoproteasome plays a critical role in the immune system’s ability to identify and neutralize foreign invaders, such as bacteria and viruses. As a specialized form of the proteasome, it facilitates the breakdown of these intruders, allowing the immune system to recognize their molecular structure and respond effectively. However, this crucial component can become problematic when it exhibits overactivity, mistakenly targeting the body’s own tissues and leading to autoimmune disorders. The delicate balance of the immunoproteasome’s activity highlights the necessity for precise regulation, making it a compelling target for therapeutic intervention in autoimmune diseases.
For many years, researchers have sought inhibitors that can selectively target the immunoproteasome without impacting other proteasome variants, which play essential roles in cellular recycling and waste processing. Inhibiting these more general proteasome functions could result in a host of side effects, potentially exacerbating the patient’s condition rather than alleviating it. Thus, achieving a selective inhibitor that acts on the immunoproteasome alone has been the holy grail of therapeutic development in this area.
Though progress has been made, traditional drug development approaches often fall short of creating tailored solutions capable of distinguishing between the various proteasome forms. The need for innovative methods that harness the complexities of naturally occurring biochemical pathways has become increasingly apparent.
Researchers led by Helge Bode at the Max Planck Institute for Terrestrial Microbiology have pioneered a promising approach that leverages synthetic biology to engineer new biomolecules specifically designed for selectively inhibiting the immunoproteasome. By manipulating bacterial substances through their natural biosynthetic pathways, the team has developed a technique that promises a more refined inhibitor, which could provide the desired specificity needed in autoimmune therapies.
Collaborating institutions, including the Technical University Munich and the University of Duisburg-Essen, have further enriched this endeavor by focusing on the creation of peptide-polyketide hybrids. These novel compounds combine the strengths of peptides—known for their specific biological activities—with polyketides, a group of compounds recognized for their pharmacological potential.
The use of XUT technology represents a significant leap in creating these hybrids. This method effectively utilizes docking sites within thiolation domains that are present in both non-ribosomal peptide synthetases and polyketide synthases. By fusing these two types of enzymes, researchers can generate hybrids that exhibit unique traits, enabling the production of more complex and effective therapeutic agents.
Notably, nature has already provided examples of such hybrids, seen in substances like syrbactins found in certain bacteria. These compounds were initially recognized for their ability to disrupt the proteasome of plant and insect cells, leading to cell death—a phenomenon that has spurred interest in their application as potential anti-cancer drugs. The insights gained from studying these natural compounds inform the design of synthetic variants that can inhibit human immunoproteasomes with greater precision.
While promising results have emerged from these research efforts, the inhibitors developed thus far have not yet achieved the level of selectivity required to minimize side effects fully. However, the ongoing research indicates a clear path to optimization, where computational tools will allow scientists to simulate and evaluate new variants quickly and efficiently. This high-throughput approach can significantly accelerate the pace of discovery, paving the way for more effective and safer treatments for autoimmune diseases.
The progress made in manipulating bacterial substances for targeted immunoproteasome inhibition signifies an exciting shift in the development of specific therapies for immune-related disorders. As researchers continue to refine their methods and enhance selectivity, the dream of precision medicine for treating autoimmune diseases is drawing closer to reality, potentially transforming the lives of millions afflicted by these conditions.