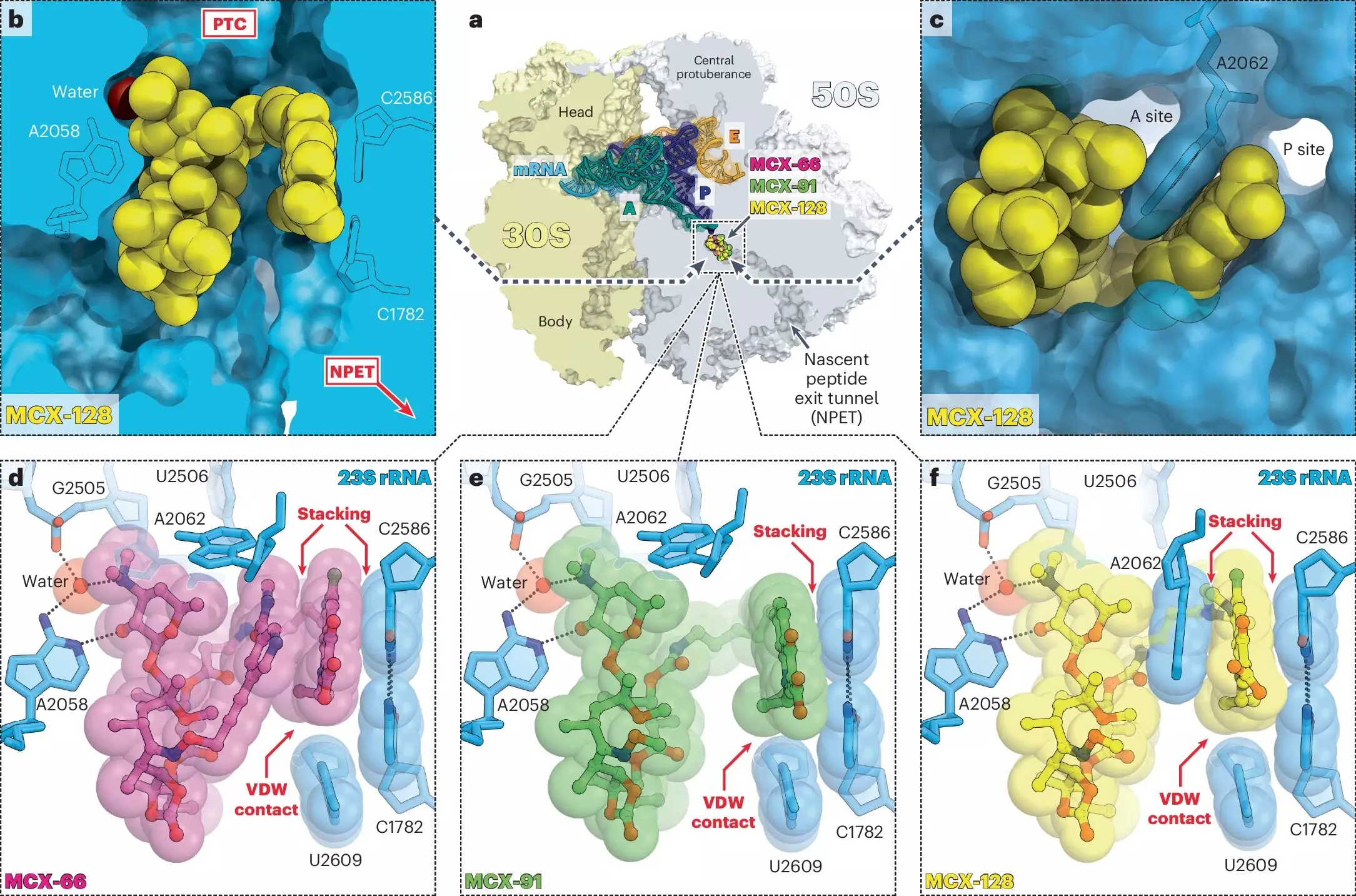
In the ever-evolving battle against antibiotic resistance, a novel class of antibiotics dubbed macrolones has emerged as a beacon of hope. Research conducted by the University of Illinois Chicago demonstrates that these synthetic antibiotics possess the remarkable capability to disrupt bacterial functions through two distinct targets. This groundbreaking approach not only enhances efficacy but renders the likelihood of bacterial resistance nearly obsolete. With a staggering potential of increasing the difficulty for bacteria to mutate and evade treatments by 100 million times, macrolones signal a significant leap forward in infectious disease management.
The Mechanism Behind Macrolones
Macrolones are ingeniously designed amalgams of two well-established antibiotic classes: macrolides and fluoroquinolones. Macrolides, such as erythromycin, inhibit protein synthesis by binding to the bacterial ribosome, effectively halting the creation of essential proteins. On the other hand, fluoroquinolones, like ciprofloxacin, target DNA gyrase, an enzyme crucial for bacterial DNA replication and repair. The dual action of macrolones—disrupting both protein production and DNA integrity—sets the stage for a more effective antibiotic that complicates the bacterial survival strategy. According to Alexander Mankin, a leading researcher in the study, this unique action does not allow for the bacteria to develop simple genetic defenses against such a dual assault.
Unprecedented Efficacy Against Resistant Bacteria
What makes macrolones strikingly unique is their ability to bind tightly to ribosomes, even in bacterial strains that have developed resistance against traditional macrolides. The research findings reveal that these new antibiotics can successfully inhibit macrolide-resistant strains without triggering the activation of resistance genes. This characteristic positions macrolones as possible frontline agents in treating infections that have frustrated healthcare providers for years. The enhanced binding affinity increases the likelihood of bacterial destruction while limiting the chances that unintended mutations will yield survival mechanisms—heralding a new dawn in antibiotic efficacy.
Collaboration at UIC: A Model for Future Research
The study also underscores the importance of interdisciplinary collaboration in advancing scientific understanding. Researchers from various fields—pharmaceutical sciences, biology, and chemistry—came together at the UIC Molecular Biology Research Building, showcasing how shared expertise can enhance exploratory work. The team, led by Yury Polikanov and Mankin, has transcended traditional silos to foster an environment where innovative ideas can thrive. This collaborative approach is not merely a contextual backdrop; it is a core component of the research’s success story. Interdisciplinary studies like this one will be vital as we navigate the complexities of microbial resistance and strive toward developing viable solutions.
The Future of Antibiotics: Redefining Resistance Management
The implications of the macrolones research extend far beyond a single study or even a series of successful lab results. The challenge of antibiotic resistance is one of the most pressing problems in contemporary medicine, and the widespread use of dual-target antibiotics may be one of the most effective strategies we have to counter this threat. As Mankin emphasizes, optimizing these macrolones to harness their full potential in targeting bacteria concurrently is critical. This future-centric vision encapsulates the goal of creating a formidable arsenal against infectious diseases—a pressing necessity in an age where conventional methods are often failing.
The promise of macrolones serves as a clarion call to the scientific community to foster an environment that values innovative designs and collaborative efforts. It is a call to action as we stand at the precipice of a new era in antibiotic therapy. We must embrace these findings, enhance our understanding, and champion the discovery of even more sophisticated solutions to combat the dark shadow of resistance that looms over global health.