In a groundbreaking revelation, researchers from the Walter and Eliza Hall Institute of Medical Research (WEHI) have made significant strides in understanding PINK1, a mitochondrial protein intricately linked to Parkinson’s disease. This research is pivotal, not only in terms of the scientific breakthrough it represents but also regarding its implications for those battling the relentless grip of this neurodegenerative disorder. Parkinson’s has long been characterized by its complex pathology, and prior to this, the precise function of PINK1 and its implications in the disease remained largely nebulous. What makes this discovery particularly compelling is the way it illuminates the pathways that govern mitochondrial health and survival in neurons—critical factors in preventing neurodegeneration.
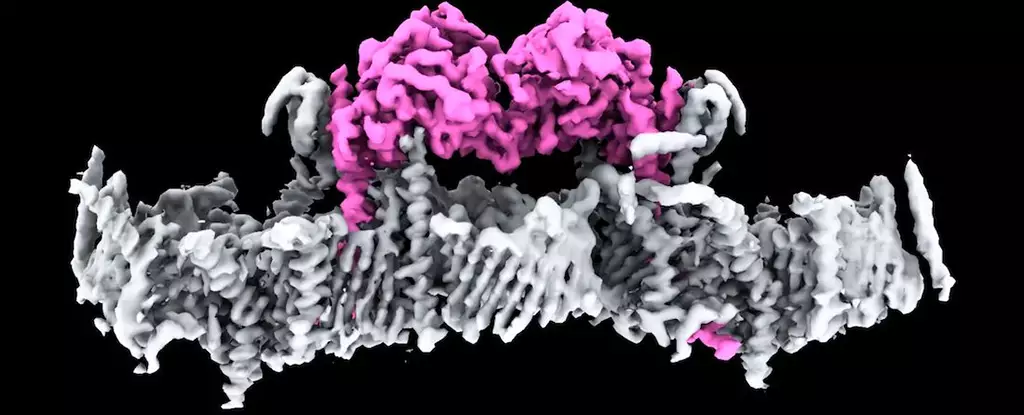
For over two decades, scientists have identified that mutations in the PINK1 gene can lead to early-onset Parkinson’s disease. Yet, the mechanisms by which this occurs have eluded researchers. Now, through advanced imaging techniques such as cryo-electron microscopy and mass spectrometry, the team at WEHI has gained unprecedented insights into the structural mechanics of PINK1. This type of study represents a major methodological leap in biochemical inquiries, allowing researchers to visualize proteins in their natural environments much more effectively than previous technologies enabled.
A Mitochondrial Maintenance Crew Member
PINK1 functions as a critical maintenance worker within the cellular energy landscape. In a healthy state, it gracefully traverses the outer and inner membranes of mitochondria, often remaining unnoticed within these powerhouses of the cell. However, in instances where mitochondria have degraded or become dysfunctional, PINK1 is forced to halt at a specific checkpoint. This is not merely random mechanics; PINK1’s pause triggers an essential tagging process, signifying these damaged mitochondria for removal via a cascade of molecular signals, predominantly through ubiquitin.
This tagging process is crucial as it allows the cell to avoid the detrimental consequences of retaining inefficient energy sources, especially in brain cells, which have demanding energy requirements. If PINK1 cannot perform its role effectively due to genetic mutations, the repercussions can be catastrophic. The failure to clear dysfunctional mitochondria leads to a relentless decline in neuronal health, setting the stage for neurodegenerative conditions like Parkinson’s disease.
Pioneering New Frontiers in Treatment
The implications of this research extend far beyond mere understanding; they reach into the realm of potential therapies. Identifying how PINK1 interacts with mitochondria lays the groundwork for developing innovative treatments that could enhance or restore the protein’s functionality. David Komander, a notable medical biologist involved in the study, described the findings as life-changing for Parkinson’s patients. The ability to “switch on” PINK1 through targeted interventions may one day transform the therapeutic landscape, offering new hope for slowing disease progression or reducing risk.
Moreover, this research also brings to light the complexities that underpin Parkinson’s disease. Biochemist Sylvie Callegari emphasized the newfound understanding of the interplay between PINK1 mutations and mitochondria. It uncovers a network of proteins that not only informs researchers but also reconstructs our understanding of disease mechanics, highlighting that Parkinson’s is not a monolithic condition but rather a spectrum of biological failures.
A Multifaceted Approach to Understanding Parkinson’s
Despite these promising developments, it is essential to note that Parkinson’s disease encompasses a multitude of contributing factors beyond just PINK1. The identification of one mechanism does not provide a panacea; rather, it opens a window into a broader dialogue regarding the interplay of genetics, environmental factors, and other proteins involved in neuronal maintenance.
As investigations into PINK1 advance, they illuminate pathways that may share commonalities with other neurodegenerative diseases. This suggests a potential for integrative approaches to understanding and treating various conditions under the umbrella of neurodegeneration. Thus, the scientific community stands on the precipice of a new frontier that could ultimately redefine not only our approach to Parkinson’s disease but also our understanding of neuronal health and longevity in the human brain.
The research validates the notion that science is often about the journey toward understanding rather than having all the answers. It emphasizes persistence, collaboration, and innovative thinking as driving forces for solving the mysteries of complex diseases like Parkinson’s, offering a glimmer of hope to millions.